Chapter 5 - Other issues raised in the inquiry
5.1
Improving cancer outcomes is a multifactorial field
that extends far beyond the scope of this inquiry. While the Committee's
investigations were necessarily focussed by the terms of reference, a number of
specific issues relating to cancer treatment and care were raised in
submissions and at the hearings. These are briefly discussed in this chapter.
Early detection through screening programs
Access to free mammograms
5.2
The issue of providing free mammograms for women outside
the target ages, through the national breast cancer screening program, was
raised.
5.3
The Department of Health and Ageing advised that BreastScreen
Australia actively targets women aged 50-69 years, but women aged 40-49 and
over 70 years are also eligible to attend.
5.4
This policy is in line with international evidence
which demonstrates that breast cancer screening is most effective in the 50-69
year age group. BreastScreen Australia
is a joint Commonwealth, State and Territory funded program. Funding is
provided by the Commonwealth through the Public Health Outcome Funding
Agreements. The Agreements are bilateral funding agreements between the
Commonwealth and each State and Territory Government. The agreements provide
State and Territory Governments with broadbanded (pooled) funding linked to the
achievement of outcomes in a range of public health programs including breast
cancer screening.
5.5
An article in the March 2002 edition of the Medical Journal of Australia reports on
outcomes of a systematic review of screening mammography in women 70 years and
over. The review concludes that:
- Age is the strongest risk factor for breast
cancer, as indicated by the increasing number of cancers detected across age
groups - however, because older women at a higher risk of death from other
causes, they may only experience the downsides of screening, and not live long
enough to experience the benefits; and
- Women aged 70 years and over, in consultation
with their doctor, may want to decide for themselves whether to continue
mammographic screening.[313]
5.6
This is consistent with current BreastScreen Australia
policy, although individual States and Territories have different practices in
relation to service provision for women over 70 years of age. All women over
the age of 70 years can make an appointment and attend any BreastScreen
Australia service across Australia.
5.7
All eligible women aged 50-69 years who already attend
BreastScreen Australia
services are reinvited to attend for breast cancer screening every two years.
However, there are differences between jurisdictions as to when women are no
longer invited to attend for breast cancer screening. Where States and
Territories do cease to reinvite after women have reached an upper age limit,
letters are sent to women affected outlining the reasons why they will not be
reinvited in the future but that they are free to call and make an appointment
for a two-yearly mammogram if they wish.[314]
Access to free mammograms once
diagnosed with breast cancer
5.8
Witnesses raised the issue of their ability to access
breast cancer screening through BreastScreen Australia
following a diagnosis of breast cancer.[315]
My breast cancer was detected by BreastScreen and I found they
provided an efficient service of the highest professional standard. So I was understandably
surprised when I was advised that my follow up mammograms would not occur at
Breastscreen, but on a referral from my surgeon to a private Radiologist...
When I made my appointment with this private radiologist, I was
informed that the mammogram and ultrasound would cost $314-00, payable at the
time of service. Fortunately at the time, I was in a position to meet such a
financial demand, but I know of many women who would find an up front payment
difficult under any circumstances. The benefit I received from Medicare for
this service was $164-60, leaving my family out of pocket $149-40. This is
obviously a huge burden for many women on low or no incomes; it is a pressing
social issue for women who have no direct access to money, which is not uncommon
when many women are forced to take long periods off work to undergo cancer
treatment plans.[316]
5.9
The BreastScreen Australia Program, as a population
screening program, is aimed at well women, without symptoms. BreastScreen Australia
services recommend that women who have had breast cancer in the past and have
had surgery to remove a lump or for a mastectomy continue to visit their breast
specialist for their regular mammograms. Reasons for this include:
- If a woman has had breast cancer and surgery to
remove a lump, special techniques and procedures may be required, such as
detailed pictures of the treated part of the breast. These special procedures
are not offered at a screening visit as BreastScreen Australia is set up to
provide mammograms to detect the apparent early signs of breast cancer in women
with no symptoms.
- If a woman has breast cancer, regular check-ups
should involve a thorough clinical examination by a doctor, annual mammograms
and any other test that may be required. BreastScreen Australia only provides
screening mammograms.
5.10
State and Territory BreastScreen Programs are
responsible for determining their own policies for making services available
for women who have been diagnosed with and treated for breast cancer. Some
States take women who have had treatment for breast cancer back into the
screening program after a specified period of time, others take such women back
if they have a letter from their treating surgeon indicating that it is
appropriate for that woman to return to biennial mammographic screening.
5.11
The Committee is also aware that the Cancer Funding
Reform Project, reporting through the Health Reform Agenda Working Group to
Australian Health Ministers is examining a range of strategic funding issues
associated with the provision of cancer care. The project will investigate the
current funding arrangements for cancer treatment in the Australian health
system across the public and private sectors.
Recommendation 30
5.12 The Committee recommends that the target age groups for
BreastScreen Australia
and the National Cervical Screening Program should be reviewed regularly, given
the increasing trends in life expectancy for Australian women. In addition, a
review should be conducted of how women outside the age limits are made aware
of their cancer risk.
Access to breast prostheses and lymphoedema sleeves
5.13
Issues relating to access to breast prostheses and
lymphoedema sleeves were raised in evidence.[317]
5.14
Ms Crossing
explained that if a women has a mastectomy they need a breast prosthesis so
their spinal alignment does not become compromised and cause other health
problems. She reported that access to breast prostheses after a mastectomy is
not consistent between States. The same difficulties apply regarding access to lymphoedema
sleeves which are necessary to treat 'painful swelling of the arm'.[318]
5.15
The Department
of Health and Ageing advised that the Medicare Benefits arrangements are
designed to provide assistance to people who incur medical expenses in respect
of clinically relevant professional services that are contained in the Medicare
Benefits Schedule, and rendered by or on behalf of qualified medical
practitioners. Therefore Medicare benefits are not payable for the costs of
aids and appliances, including breast prostheses and lymphoedema sleeves.
5.16
However, the Commonwealth does provide funding for the
surgical implantation procedure, under the Medicare Benefits Schedule for
privately insured patients (excluding those seeking implantation for purely
cosmetic purposes) and for public patients (including the prostheses) through
the Australian Health Care Agreements.
5.17
The Plastic and Reconstructive Subgroup of the Medicare
Benefits Schedule contains a number of services which provide for the surgical
implantation, removal and/or replacement of breast prostheses as well as breast
reconstruction procedures for women who have undergone mastectomy.
5.18
In addition, private health insurance funds are
currently required under the Surgically Implanted Prostheses, Human Tissue
Items and Other Medical Devices (Schedule 5) of the National Health Act 1953 to
fully fund prostheses items that are provided as part of an episode of hospital
care, such as breast implants.
5.19
The funding for breast implants listed in Schedule 5 is
limited to patients who have undergone specific Medicare Benefits Schedule
procedures; it does not cover the prostheses provided for cosmetic procedures
such as breast enlargement. The range of surgically implanted breast prostheses
listed on the Prostheses Schedule includes both saline and silicone-gel filled
prostheses. The Commonwealth has had no role in the funding of products currently
listed on Schedule 5. This has been a matter between the health funds and the
supplier of the product.
5.20
For external prostheses (not surgically implanted) like
breast prostheses and lymphoedema sleeves, private health insurance funds may
be able to provide a rebate for the cost of the prostheses as part of their
ancillary cover.
5.21
If the person does not have private health insurance,
help may be available from State/Territory governments, such as the Program for
Aids for Disabled People in New South Wales.
Access to PET scans for people with recurrent or advanced breast cancer
5.22
The issue of access to Positron Emission Tomography (PET)
scans for people with recurrent or advanced breast cancer was raised.[319] Ms Crossing noted that although Positron
Emission Tomography scans for most cancers are funded by the Commonwealth, they
are not funded for breast cancer even though a great deal of evidence shows it
is an important tool for following and staging the course of advanced breast
cancer. Cost implications for the patient are significant with Ms Crossing
indicating that 'it is $900 out of your pocket and that is a huge sum of money
for most women faced with this particular situation'.[320]
5.23
The Department of Health and Ageing advised that there
are currently 13 PET scanners in Australia.
Nine scanners receive Commonwealth funding in eight facilities and all eight facilities
are participating in an evaluation of PET clinical and cost effectiveness.
Results from this program are expected to become available from mid 2006 and
will inform the decisions about future PET funding.
5.24
The Department also advised that the average Medicare Benefits
Schedule fee for a PET scan is around $950 and that conditions of Commonwealth
funding specify that scans are performed at no or minimal out of pocket cost to
the patient. PET effectiveness and cost effectiveness in the management of
breast cancer would need to be considered by the Medical Services Advisory
Committee before any decisions about public funding could be made. The role of
the Medicare Service Advisory Committee is to advise the Federal Minister for
Health and Ageing on the evidence relating to the safety, effectiveness and
cost effectiveness of new medical technologies and procedures.[321]
Adolescent cancer care
5.25
The provision of appropriate cancer care services for
adolescents and for young adults with cancer, an age group for whom the
incidence of cancer is increasing, was raised in evidence by a number of people
including witnesses from CanTeen and the Centre for Children's Cancer and Blood
Disorders at Sydney Children's Hospital:
Published Australian data, which mirrors overseas data,
indicates that during the past decade alone cancer incidence has increased by
30 per cent in young people aged between 10 and 24 years. This increased
incidence of cancer in adolescents and young adults is higher than in any other
age group.[322]
5.26
The Committee was advised that there is also growing
concern internationally for the adolescent and young adult cancer population
and mounting evidence for targeting improvements for this patient group.[323]
5.27
While cure rates both for younger children and older
adults with cancer have improved, the same is not true for adolescent cancer
patients as shown in Figure 5.1.
Figure 5.1: Management of cancer in adolescents
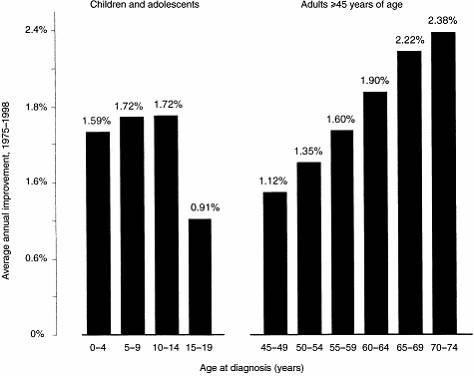
Source: Albritton K and Bleyer WA, 'The management of cancer in the older adolescent', European Journal of Cancer vol.39 2003,
pp.2584-2599.
5.28
Issues for adolescents as described for the Committee include
firstly, that access to clinical trials for adolescents with cancer is very
poor which means they are less likely to have access to state-of-the-art
treatment. Secondly, they are less likely to be treated in specialised
multidisciplinary cancer care units where the best results are achieved. There
are no guidelines for the referral of adolescents and young adults with cancer
to specialist care which means they are randomly referred to either paediatric
or adult cancer physicians. Dr O'Brien
indicated that:
Survival rates for children with leukaemia or cancer are higher
when treatment is supervised by a tertiary paediatric cancer centre, where
treatment is planned and supervised by a multidisciplinary team comprising both
medical, surgical and radiation oncological disciplines, and where treatment
utilises active trials conducted by international paediatric cooperative
groups.[324]
5.29
A recent Victorian study was quoted as demonstrating
that treating adolescents with a particular type of bone tumour in a paediatric
regime improved survival rates by 50 per cent.[325] Other evidence based on studies by
McTiernan in the UK
and referred to by witnesses reported that international studies have shown
significant improvements in outcomes for adolescents and young adults treated
on clinical trials. The review by McTiernan confirmed that adolescents with
acute lymphocytic leukaemia, non-hodgkins lymphoma, nephroblastoma and
rhabdomysarcoma as well as medulloblastoma have all shown a significant
survival advantage when treated on trial protocols within specialist centres,
compared to those that are not.[326]
Recommendation 31
5.30 The Committee recommends that Cancer Australia
consider the development of appropriate referral pathways that take account of
the particular difficulties confronted by adolescents with cancer.
Damien's story –
The needs of adolescents
In April 1999 I was diagnosed with a bone cancer
called Osteosarcoma, in my left knee. At the time I was 15 years old and was
treated at the Royal Children’s Hospital in Melbourne for 9 months.
When I was diagnosed I was a typical 15 year old. I
was very fit and healthy and had no history of cancer in my family. I didn’t
know anything about cancer or any of the treatments for it. It was something I
had never come across before.
From the time ‘something showed up on the x-ray’ until
the time I finished my treatment I wasn’t at school. This meant that I didn’t
get the opportunity to spend time with my friends like a ‘normal’ teenager
would. Even outside of school my friends didn’t come and visit me. I assume
because they didn’t know what to say. This meant that I didn’t have anyone who
was my age that I could talk to about what was happening to me. Even within the
hospital there were very few teenagers of my age due to that fact, I was being
treated at a children’s hospital...
Soon after I finished my treatment, I attended a camp
for Patient Members of CanTeen. This was the first opportunity I had had to
talk to people my age about what had happened in the last 9 months of my life
and how it would effect the rest of my life. I got to meet people who had ‘been
there and done that’ and see how they had continued with their lives...
I believe it is hard enough for a young person to grow
up and cope with the normal changes that happen in their life. Throw in a
diagnosis of cancer, and it throws the young person out of their normal life,
and into hospital. Being able to talk to people my age that had been through
similar experiences was able to bring back some kind of ‘normalness’ into my
life.
Submission 51, p.14 (CanTeen Australia).
5.31
CanTeen, the organisation that supports young people
living with cancer, indicated that 12-24 year olds undergoing treatment were
not surviving as well, or being supported as well, as were children between the
ages of 1-12.[327]
5.32
The psychosocial care needs of adolescents with cancer
differ from those of an adult or a child and are not being addressed. Witnesses,
including young patients, emphasised that when you are a teenage patient, being
treated in a children's environment adds to the frustrations in terms of the
physical facilities and the support services. These frustrations are similar if
the adolescents were treated in a ward with very sick adults. The personal
experience of a teenage cancer patient being treated in a children's hospital
was described by Ms Michels:
The women's and children's hospital has a toy room, a great
resource for little kids. The walls are painted with huge bright murals of
clowns, fairies and under-the-seas themes, all directed at small children. The
prints in the rooms are of kittens and Peter
Rabbit, and the video collection had much to
be desired. Once you have sifted through the Wiggles and stories like that, you
might get to view something like Toy Story. I wanted a couch to sit on and play
music that I like listening to. I found myself spending a lot of time in the
'quiet room', which is a room with two couches and no bright paintings or
anything. The small children did not go in there as it was not exciting.[328]
Ms
Michels then described her experience when
treated in an adult hospital a few years later:
I met a lot of lovely people and their families but I struggled
a lot because of the age gap. I did not feel like we could talk about the stuff
that teenagers talk about in front of adults. It was hard for my friends to
stay positive around me as I was surrounded by sick and older people lying in
beds.[329]
5.33
Witnesses told the Committee there appeared to be
inflexibility in decisions and policies as to where adolescents are treated
which could impact on outcomes. One example was given for NSW: 'if you are aged
15 and 11 months then you can go to a children's hospital. If you are 16 then
you cannot be admitted to a children's hospital for a new diagnosis of cancer'.[330] Ms
Ewing noted that the Cancer Control Network:
acknowledges that adolescents with cancer "present a
challenge that is not adequately addressed by current systems or models of care
in Australia".
[White 2002]. This situation is likely to have occurred because the care of
adolescent patients is often seen as neither the preserve of paediatric or
adult services [Leonard et al 1995], and consequently these people fall into
the void between.[331]
5.34
It was suggested to the Committee that the way to
address this issue is for the establishment of specialised teenage cancer units
where there could be collaboration between both paediatric and adult cancer
specialists. Such a unit would utilise a multidisciplinary team to deliver
appropriate medical and psychosocial care.[332]
The needs of
adolescents
The needs of adolescents are different to those of
both children and adults, as there is this middle ground. We are not dependent,
like children are on their parents, but we do not have people dependent on us.
We have all different issues. By having adolescent wards you would be
surrounded by people where you fit in, you feel like you belong and you are not
alone. You could have the same interests. Friendships would naturally form and
support would be given. Adolescents would be surrounded by others that are
dealing with similar situations in and out of hospital. They can relate to what
is going on, as they are going through the same things. There would be a
positive environment with others who they can feel comfortable and relaxed
amongst. We can share, listen, have fun, joke, be ourselves, relax, learn, heal
and grow throughout this. Talking is a great healer for cancer patients because
it releases disturbing thoughts bottled up inside. It is proven beyond a doubt
that the mind can help heal the body when you are thinking positively. Cancer
patients and other young people living with cancer have a genuine understanding
of each other’s situation and what we are going through.
Committee Hansard 19.4.05, p.63 (Miss
Lauren Michels).
5.35
The Committee concluded that it was very important that
information was provided as soon as possible about the current treatment
profile in Australia
for this age group, how it compares with other countries and how many clinical
trials are available and being accessed. In terms of the environments in which
these young people are being treated, often for long periods of time, it was
important to ask the State and Territory health departments how they are addressing
the issue.
Recommendation 32
5.36
The Committee recommends that State and Territory
Governments recognise the difficulties experienced by adolescent cancer
patients being placed with inappropriate age groups and examine the feasibility
of establishing specialised adolescent cancer care units in public hospitals.
Research
5.37
The Committee noted that cancer research in Australia
is funded by a number of bodies including the Commonwealth, through the NHMRC,
as well as State and Territory governments, Cancer Councils and charities and
others.
5.38
The Commonwealth Government announced as part of the
2005-06 Budget that funding will be provided over four years for a dedicated
cancer research budget and that seed funding is to be provided to establish a National
Research Centre for Asbestos Related Diseases.
5.39
The Committee was advised that the Cancer Institute
New South Wales has a major research program and has invested research
fellowships, infrastructure that enables researchers to access equipment and expertise,
and translational (bench to bedside) research.[333]
5.40
The Victorian Department of Human Services has
established a Cancer Research Working Group. The group provides advice on the
better integration, coordination and development of cancer research and promotes
communication between research centres and health services to facilitate the
translation of cancer research into clinical practice.[334]
5.41
The Cancer Institute New South Wales
has recommended that the Commonwealth government consider a more strategic focus
for cancer research.[335] The Institute
suggested that, in addition to the traditional areas funded by the NHMRC,
further research should be directed towards translational research, health
services research, screening and early detection, and clinical trials.
Clinical Trials
5.42
Clinical trials are fundamental to establishing whether
there is benefit in new treatments. Participation in clinical trials needs to
be encouraged as there is evidence that people receive better care and have
longer survival if enrolled in trials, though there is a considerable disparity
between the numbers enrolled.[336]
5.43
Witnesses expressed concern at the relatively low
enrolment of people in clinical trials, with the enrolment of adults being
around two to three per cent though 20 to 30 per cent are eligible.[337] This contrasts with children, where
every child is considered for a trial and over 50 per cent are entered.
5.44
The Cancer Institute New South Wales
stated that national cancer clinical trials are poorly funded and operate on
grants from philanthropy. The Institute called for the provision of support to
these groups from governments throughout Australia.[338]
5.45
The Committee noted that in response to the low
enrolments in clinical trials, the Cancer Institute New South Wales
established The Clinical Trials Program which has four main aims, to:
- Introduce and study new cancer treatments;
- Increase participation rates in cancer clinical
trials;
- Promote a culture of research and innovation in
our cancer service programs; and
- Connect cancer clinical trials in New South
Wales to key national and international trials.
5.46
A Clinical Trials Office has been established to assist
the Cancer Institute New South Wales
in achieving the above listed aims. The Clinical Trials Office will endeavour
to provide high quality cancer clinical trial infrastructure for New
South Wales, managing the initiatives identified as a
result of workshops and discussions with key stakeholders and groups.[339]
5.47
The Committee also noted that the Commonwealth is
committing significant funding over the next four years to provide infrastructure
grants for cancer clinical trials through the Strengthening Cancer Care
Initiative.[340]
Data
5.48
Many witnesses identified gaps in cancer data, which if
addressed could lead to improvements to both service planning and treatment for
cancer patients.
5.49
The Australian Institute of Health and Welfare
identified three major gaps in national data on services and treatment options.
5.50
The first related to the lack of national data on
hospital outpatient services for cancer. The AIHW indicated that from July 2005
a collection of hospital outpatient occasions of service delivery for
chemotherapy and radiation oncology would commence for the principal referral
and other major hospitals in each State.
5.51
The second area is data on the stage of cancer, a
pre-requisite to interpreting changes in survival and to analysing the effects
of changes in treatment and services. The need for staging data was also
strongly supported by Dr Threlfall,
Manager and Principal Medical Officer, Western Australia Cancer Registry.[341] The AIHW has acknowledged that some
work was occurring in the area of staging data. For example:
- The National Cancer Control Initiative has
developed a national clinical cancer core data set. The data set has been
endorsed by the National Health Data Committee of the Australian Health
Ministers Advisory Committee. The National Cancer Control Initiative has also
undertaken some pilot work in Western Australia and the Northern Territory on
the feasibility of collecting staging data.[342]
- The Cancer Institute New South Wales has
commenced a program for the collection of a minimum data set of 45 items on
every cancer patient in New South Wales. The minimum data set is targeted at
the patient's journey and is expected to be rolled out within a 12 month
period.[343]
- The Victorian Department of Human Services has
established a Data/Information Working Group that is promoting the collection
of the National Cancer Control Initiative's Minimum Data Set.[344]
5.52
The AIHW acknowledged that the standardisation of
staging data across States and Territories would take some time and that the
coding from the detailed to the aggregated data set would be very costly.[345]
5.53
The third gap relates to the lack of linkage between
existing data sets. There are a number of individual data sets from the
Medicare Benefits Schedule and Pharmaceutical Benefits Scheme, the Health
Insurance Commission data bases, and hospital records, where there is little
linkage of these data bases. Commonwealth statisticians and the ABS are working
on protocols on how the ethical linkage of data can be undertaken, taking into
account relevant privacy legislation.[346]
5.54
The Committee heard that there was a major gap in the
collection of data on cancer staging. Data on the stage of cancer is a
pre-requisite to interpreting changes in survival and to analysing the effects
of changes in treatment and services. The AIHW acknowledged that while work is
being undertaken in this area, the standardisation of staging data across
States and Territories would take some time and would be costly. Given the
importance of cancer staging data, the Committee makes the following
recommendation.
Recommendation 33
5.55 The Committee recommends that Cancer Australia,
in consultation with State and Territory Governments and the Australian
Institute of Health and Welfare, take a leadership role in coordinating the
development of a national approach to the collection of cancer staging data.
Palliative care
5.56
Palliative care was raised by a number of witnesses as
an area requiring greater attention due to the increasing incidence of cancer.
As stated by Professor Kricker
'the fact that 36 000 die is not reflected in the state of development of
the palliative care services. It is a crying need'.[347]
5.57
Palliative care is a relatively new discipline in Australia's
health care system and aims to improve the quality of life of people with
life-limiting conditions.[348] To a
great extent, hospice palliative care in Australia
has been driven by community demand through non-government organisations led by
doctors, nurses, other committed health professionals and members of the
public.
5.58
The provision of good palliative care is not just for
the benefit of the terminally ill patient. Providing good palliative care at
the end of a cancer patient's journey has measurable health outcomes in terms
of the unpaid carers. Professor Currow
stated:
I would like to reflect on the fact that good palliative care is
not a black hole into which we pour money; it is something with measurable
health outcomes that are felt long after the death of a person. The care giver
impact is positively affected by the involvement of palliative services and
that effect has hangover, if you will, that lasts for many years after the
death of the person who has had a life-limiting illness. The very small
investment that we make in palliative care has an enormous benefit for the
health of the whole community when measured in those sorts of parameters.[349]
5.59
It is evident from the growth in the demand for
domiciliary palliative care throughout Australia that patients in the final
stage of the cancer journey appreciate the option of being able to die at home
or, at least, spend as much time as possible there. Dr
Helen Manion
reported that a World Health Organisation survey showed that 80 per cent of
people have the wish to be able to remain in their own homes to die but the
reality is that the majority of cancer patients die in an institution.[350]
5.60
Despite improvements in survival rates, Professor
Currow stated that 'one in two people diagnosed
with a solid cancer will still have their life substantially shortened by that
in 2005. So we need excellent support and end of life care'. Currently the
majority of patients (greater than 80 percent in most cases) referred to
palliative care services in Australia
have cancer.[351] Given the increasing
numbers of people with cancer, without recognition of this resource need, the
planning of future cancer services across the country will continue to be ad
hoc. Professor Currow stated that 'unless we start to plan for the future in a
very proactive way and ensure that every position has the flow-on effects of
all the allied health, nursing and medical needs – and equalling that with the
challenge of ensuring that we are providing infrastructure across the continuum
of care; so in the community, in in-patient settings and in out-patient
settings – we are going to have problems in the future'.[352]
5.61
Professor Currow
mentioned the variation across Australia
in metropolitan, rural, regional and remote Australia
in accessing specialised palliative care services.[353] This disparity was highlighted by
investigators at the University of Western Australia who conducted research
which found that one third of people who died of cancer had not receive
specialist palliative care. They found that people were less likely to receive
specialist palliative care services if they were aged 84 years or over; female;
Aboriginal; living in remote areas; or socioeconomically disadvantaged. Their
research also found that use of specialist palliative care services reduced the
likelihood of dying in a hospital or in a residential aged care facility,
suggesting that the use of specialist palliative care services potentially
reduces the demand on other hospital beds.[354]
5.62
Submissions suggested that the use of care coordinators
is important to ensure that all patients are referred to a specialist
palliative care service.[355]
5.63
The role of the carer of a terminally ill patient has
been recognised by the Australian government. Some carers qualify for financial
entitlements through Centrelink with the Carer Payment for those who are not
able to work due to their caring responsibilities and the Carer Allowance,
which helps parents or carers to care for adults with a disability at home.[356]
5.64
The Commonwealth is 'providing $201.2m throughout the
five years of the Australian Health Care Agreements (2003-08) for palliative
care. Of this, $188m is broadly allocated on a per capita basis to States and
Territories for continued service provision, and $13.2m for the Australian
Government to implement a national program of initiatives. In the 2002 Federal
Budget, the Australian Government announced a further $55m over four years
(2002-06) for national activity to improve the standard to palliative care
offered in local communities'.[357]
5.65
To meet the demand for palliative care in the home,
witnesses raised concerns over the availability and supply of some drugs.[358] Drugs that are available in a
hospital are not automatically available for a patient being looked after at
home. The Pharmacy Guild recommended that 'the range of medication used in
palliative care listed in the PBS be broadened to assist in providing wider
access to medication at an affordable price for patients who wish to remain in
the community during the terminal phases of their lives'. The Guild
acknowledged that there have been recent listings of several medications but is
concerned that preparations currently listed are not adequate, citing Midazolam
and Ketalar as examples. They explained that 'there is little incentive for
manufacturers to apply for PBS listings for these drugs for innovative uses
such as in palliative care' and recommended that the dual listing of
medications used in palliative care should be investigated.[359]
5.66
The Committee questioned the Department of Health and
Ageing about why a drug that has gone through an approval process for a
specific reason, and when it may then need to be used in a different dose or in
a different treatment, needs to go through the process again as it is very
expensive and there is marginal, if any, profit for the manufacturer to do it.
5.67
In response to this issue about drugs on the palliative
care list, Dr Lopert from the Department of Health and Ageing, advised that
they are 'aware that there is concern over availability of some medications on
the palliative care list, but their lack of availability of the palliative care
list reflects that fact that they do not have marketing approval for the
indications that are relevant to the palliative care setting'. Dr Lopert stated
that the process is a safeguard as 'the broader issue from the PBS point of
view as opposed to the registration point of view is that it is inappropriate
to provide reimbursement for drugs for an indication outside that for which it
is approved for marketing in Australia – that is one of the principles
underlying the PBS...The issue of approval for indications other than those for
which it is registered is an issue for the TGA rather than the pharmaceutical
benefits branch'. When questioned specifically about Midazolam, Dr
Lopert stated 'the approved indication is
actually quite narrow. It is not approved for an indication that could be
conceivably appropriate for use in a palliative care setting – it talks about
use as an adjunct in anaesthesia for a surgical procedure'.[360]
5.68
The Committee noted there was some confusion about the
authority process for palliative prescriptions, particularly for the first
dose. The Department of Health and Ageing provided the following advice:
Requirements for prescriptions for palliative care medicines to
be authorised by the Health Insurance Commission were put in place to minimise
use outside the intended population whilst ensuring access to patients with the
greatest need. It would be impractical to not require authorisation of the 'initial'
supply, whilst requiring authorisation for continuing supply. First, without
the authority mechanism it would not be possible to monitor where an initial
supply has occurred. Second, it is most likely that medical
practitioners would continue to prescribe under the 'initial' supply
arrangement without ever seeking authority to prescribe a continuing supply.[361]
5.69
The Department also advised that the work of the
Palliative Care Medications Working Group continues, with a further medication,
Paracetamol Sustained Release, included on the palliative care section of the
PBS in April 2005. A further list of 10 medications have now been
prioritised by the Palliative Care Medications Working Group, and will be
progressed for listing in coming months. For example, Flinders University of
South Australia has now been engaged to support the generation of evidence and
data to support registration and listing of these medications under the Scheme.
In addition to the above, the Working Group is working on a number of strategies
to: support quality use of medications through education and support for GPs
and other primary health care workers, in the
management and care of palliative care patients in the community; and increase
the awareness of health professionals and the broader community on medications
currently available and how they can be accessed.
5.70
Dr Page
raised the issue of access to palliative care in regional and rural areas. She
stated that 'palliative care and pain management is becoming an increasingly
specialised field which, again, translates very poorly into rural and remote
areas. I am very distressed to say that the worst palliative care services are
often for children's cancers'. Dr Page
also stated that 'palliative care is something which should be available in
every country town' and highlighted 'there are a vast number of GPs out there
with palliative care skills and advanced level pain management skills'.[362]
5.71
Palliative Care Australia,
the peak national organisation representing palliative care in Australia
released a detailed set of standards for providing quality palliative care for
all Australians on 23 May 2005.
Workshops to promote and explain the new standards are currently underway
throughout the country.
5.72
The Committee noted that as the Australian population
ages and the incidence of cancer increases, the community's need for quality,
long-term palliative care will grow. It is essential that the health care
system (including public, private and not-for-profit) is well equipped to
provide quality palliative care services that meet new national standards.
Navigation: Previous Page | Contents | Next Page