Chapter 4 - Coordinated Care Trials
4.1
This chapter discusses the
evolution and development of the Coordinated Care Trials and the effectiveness
of the trials in achieving better health outcomes for clients and in improving
service delivery. The purpose of the Coordinated Care Trials is to test whether
multi-disciplinary care planning and service coordination leads to improved
health and well-being for people with chronic health conditions or complex care
needs. Funds pooling between Commonwealth and State/Territory programs was
trialed as a means of providing funding flexibility to support this coordinated
approach to service delivery.[138]
Development of Coordinated Care Trials
4.2
The Coordinated Care Trials
were developed in response to a Council of Australian Governments endorsed
reform agenda in April 1995 that sought to meet Australia’s
health care needs in more appropriate ways while managing health expenditures
more effectively.
4.3
The then Department of Human
Services and Health called for expressions of interest in September 1995 to
establish trials that would develop and test innovative service delivery and
funding arrangements. Nine trials were approved by the Commonwealth and clients
were recruited from July 1997. Due to the complexity of the design phase, slower
than expected rates of recruitment and developments within the health system
that affected the trials and necessitated changes to their design, the
scheduled end date for the trials was extended to 31 December 1999.[139]
The coordinated care model
4.4
The coordinated care model
consists of:
-
a trial sponsor (such as an Area Health Service
or Division of General Practice) which is contracted to Commonwealth and State
governments to manage the trial;
-
a funding ‘pool’ which combines funds drawn from
a range of Commonwealth and State health care programs such as the MBS, PBS,
Home and Community Care Program and hospital funding. These funds can be used
to buy any services for individual patients considered appropriate;
-
a care coordination process which can be undertaken by a person (a local GP, a
community nurse or designated coordinator), or a service (such as an Aged Care
Advisory Team); and
-
a defined client group (usually people with high
care needs with a particular diagnosis or condition, or those with a range of
chronic illnesses). [140]
4.5
The organisational and funding
arrangements for the trials are shown below.
Figure 4.1: Organisational arrangements for trials
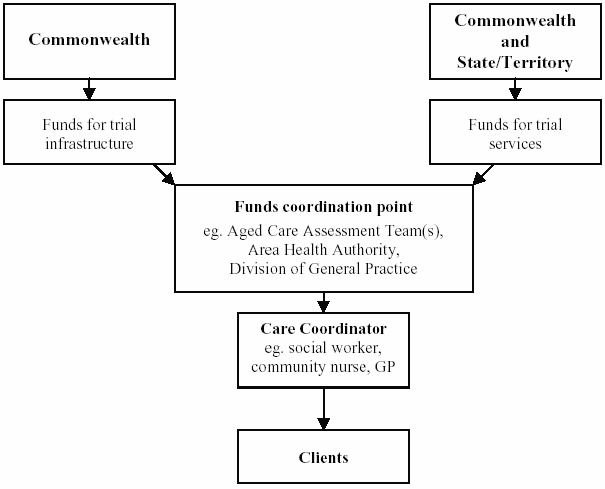
Source: Parliamentary Library, Coordinating Care in an Uncoordinated
Health System, May 1999, p.5.
4.6
The primary purpose of the
trials was to develop and test different service delivery and funding
arrangements, and to determine the extent to which the coordinated care model
contributes to improved client outcomes; better delivery of services which are
individually and collectively more responsive to clients’ assessed needs; and
more efficient ways of funding and delivering services.[141] As noted above, the Commonwealth and
the States pooled their funds for health and community services for each of the
trials’ participants. Infrastructure costs, relating to costs associated with
IT systems, office accommodation and evaluation costs were in some cases shared
between the Commonwealth and the States and in other cases were funded solely by
the Commonwealth. Although initially considered for inclusion, residential aged
care programs, such as nursing homes and hostels, were excluded as the funding
could not be easily transferred into the pool because these services are often
privately operated.[142]
4.7
Each trial had its own pool
that it had to manage in order to provide the best care possible for its
clients. The amount of money placed in each pool was based on an estimate of
what would otherwise have been spent on services used by clients who were participating
in the trials. Once an estimate for a trial client’s needs for a particular
service was calculated, that is, their needs were they not to enter the trial,
these funds were typically notionally allocated to the trial and funds
transferred monthly. Providers then billed the trial, or in the case of the MBS
and PBS, the Health Insurance Commission, and funds flowed between the trial
and the providers.[143] The following
table shows the fund pool income for each trial by item.
Table 4.1: Coordinated Care Trials:
fund pool income
|
|
Care 21
|
Care Net
|
Care Plus
|
Care
Works
|
Health
Plus
|
Linked Care
|
North Eastern
|
Southern HCN
|
TEAM
Care
|
|
MBS income
|
507 171
|
2 068 893
|
1 174 402
|
593 479
|
2 487 751
|
1 003 427
|
625 963
|
2 545 408
|
926 674
|
|
PBS income
|
487 197
|
1 825 086
|
763 966
|
502 342
|
1 544 598
|
878 971
|
505 600
|
1 164 850
|
731 454
|
|
DVA income
|
194 466
|
844 587
|
0
|
614 198
|
0
|
511 675
|
310 033
|
0
|
894 696
|
|
Hospital income
|
1 669 054
|
3 086 322
|
1 526 741
|
3 066 280
|
4 563 630
|
2 287 458
|
1 047 946
|
3 862 957
|
225 000
|
|
HACC income
|
437 500
|
247 122
|
531 088
|
585 353
|
481 685
|
985 883
|
496 888
|
0
|
0
|
|
RDNS income
|
267 905
|
0
|
0
|
152 459
|
638 512
|
683 753
|
219 435
|
53 973
|
0
|
|
Other income
|
0
|
0
|
0
|
0
|
0
|
71 738
|
0
|
0
|
0
|
|
Total
|
3 563 293
|
8 072 010
|
3 996 197
|
5 514 111
|
9 716 176
|
6 422 904
|
3 205 865
|
7 627 188
|
2 777 824
|
Source: DHAC, The Australian Coordinated Care Trials: Interim Technical National
Evaluation Report, 1999, p.122.
4.8
Each client in a trial had a
care coordinator who worked with the client to develop a care plan. The care
coordinator then drew on money from the funding pool to buy the full range of
services set out in the care plan. The actual process of care coordination was
open to the trials to determine. The care coordination function incorporated
the assessment of clients, care planning process and care plan implementation,
monitoring and review. The three main models were:
-
Model 1: the GP approach - under which the
client’s GP undertook all tasks associated with the care coordination
role;
-
Model 2: the GP care coordinator with the
service coordinator approach - under which the GP functioned as the care
coordinator and was supported by a service coordinator who acted as an agent or
organiser for the GP with various delegated tasks such as implementation of the
care plan through the arrangement of services;
-
Model 3: the non-GP care coordinators approach -
under which the tasks were undertaken by specifically designated coordinators
who were not GPs.[144]
4.9
While some trials adopted one
of these approaches, others used a combination of approaches.[145]
4.10
There were nine general trials
operating in 5 States and the ACT. The Trials were: North Eastern Health Care
Network (Vic), Southern Health Care Network (Vic), HealthPlus (SA), Care 21
(SA), Care Net Illawarra (NSW), Linked Care (NSW), TEAMCare (Qld), Careworks
(TAS), Care Plus (ACT). The nine trials recruited a total of 16 533
clients with complex and chronic health needs. While the characteristics of the
target population varied slightly across the trials, clients were predominantly
older persons, aged over 65 years of age, who were socio-economically
disadvantaged. The trials had over 2000 GPs involved in their operation.[146] The main features of the general
trials are shown below.
Table 4.2: Coordinated Care Trials -
main features
|
Trial name
|
Location
|
Client Eligibility
Criteria
|
Target population
|
|
|
|
Age
|
Other Criteria
|
|
|
Care21
|
Northern suburbs of Adelaide, SA
|
65+
(55+
ATSI)
|
Complex health care needs, multiple
community/health service usage
|
1200
|
|
Care Net
|
Illawarra area of NSW
|
65+
(45+
ATSI)
|
At risk of falling and/or needing multiple
services
|
1800
|
|
Care Plus
|
ACT
|
All
|
Complex care needs, high users of health
services
|
2400
|
|
Careworks
|
Southern Tasmania
|
65+
|
Complex care needs requiring multiple
health services
|
1200
|
|
Linked Care
|
Hornsby & Ku-ring-gai areas of
Sydney, NSW
|
All
|
Chronic/complex care needs including
elderly and people with disabilities
|
1500
|
|
North Eastern HCN
|
North-eastern suburbs of Melbourne, VIC
|
65+
|
Diseases/disabilities typical of older
age (eg stroke, respiratory, cardiac)
|
1600
|
|
HealthPlus
|
Central, southern and western suburbs of
Adelaide and the Eyre Peninsula, SA
|
18+
|
Condition specific project criteria (eg
diabetes) or complex, chronic care needs
|
6000-8000
|
|
Southern Health Care Network
|
Outer suburbs of south-east Melbourne,
VIC
|
All
|
Greater than $4000 hospital episode(s) over
2 year period
|
2500-3000
|
|
TEAMCare Health
|
Northern suburbs of Brisbane, QLD
|
65+
(50+ ATSI)
|
Multiple service needs
|
3000
|
ATSI
- Aboriginal and Torres Strait Islanders
Source: DHAC, The Australian Coordinated Care Trials - Background Trial
Descriptions, 1999, pp.9-11.
Aboriginal and Torres Strait Islander trials
4.11
In addition to the general
trials, there are trials for Aboriginal and Torres Strait Islander people. The
Aboriginal Trials have a somewhat different focus, arising from the importance
of comprehensive primary health care and community involvement in addressing
health needs of Aboriginal and Torres Strait Islander people.
4.12
The main purpose of Aboriginal
trials is to develop and assess innovative service delivery and funding
arrangements that are based upon community and individual care coordination
through pooling of funds from State and Commonwealth agencies. Aboriginal
Trials share many of the features of the general trials but there are some
important differences. Most are funded in respect of an entire community rather
than chronically ill individuals. MBS and PBS equivalent contributions to the
funding pool are at national average rates rather than an estimate of what
would otherwise have been spent on services, in recognition of historically very
low levels of MBS and PBS usage by Indigenous clients. Greater emphasis is
given to empowering communities as well as individuals to take control of their
own health needs. All Aboriginal trials
are implementing generic and individualised care plans with their client
populations as well as initiating new population health programs dealing with
issues such as diabetes and antenatal care.[147]
4.13
There are 4 Aboriginal
Coordinated Care Trials in 2 States and the Northern Territory: Wilcannia (Far
West Ward Aboriginal Medical Service) (NSW), Tiwi Islands (Tiwi Health Board
and Territory Health Services) (NT), Katherine West (Katherine Health Board,
Territory Health Services) (NT), Perth/Bunbury (a two site trial, Derbarl
Yerrigan Health Service, South West Aboriginal Medical Service, Health
Department of Western Australia) (WA).[148]
4.14
While the formal phase of the
Aboriginal trials is finalised, the trials will continue to receive funding by
the Commonwealth and States/Northern Territory during 2000 under transitional
arrangements after which the future of the trials will be determined.
Additional Coordinated Care Trials
4.15
The 1999-2000 Federal Budget
allocated $33.2 million to additional coordinated care trials over the next
four years to focus on people with chronic or complex care needs, with a
particular emphasis on older people who are chronically ill or disadvantaged.[149]
4.16
As with the current coordinated
care trials, the additional trials will be developed in collaboration with key
stakeholders, including State and Territory Governments, the medical profession
and other service providers, the non-government and charitable sectors, and the
private health sector. On 4 August 1999, all Australian Health Ministers
endorsed strategic directions for the additional coordinated care trials.
4.17
The primary purpose of the
additional trials is to build on the lessons of the current Coordinated Care
Trials, and further develop and test different service delivery and funding
arrangements. The trials are expected to run for three years. Trials
participating in the first round will have the opportunity to compete in the
second round of trials.
Evaluation of the trials
4.18
As noted previously, the aim of
coordinated care is to achieve better health and well-being for clients within
existing levels of resources (except for Aboriginal trials where increased
resources can also be a feature). The purpose of the trials is to test
different approaches to achieving this. Given this aim, a comprehensive
evaluation is a critical part of the program.[150]
Major interim evaluation reports on the general trials were published in
September 1999 and a final evaluation report is due in February 2001.[151] An
evaluation report on the Aboriginal trials is due in December 2000.
4.19
The Department of Health and
Aged Care (DHAC) stated that for the Aboriginal trials, all have implemented
public health and health service delivery initiatives targeting priority needs
of communities, with the aim of improving health outcomes. There are early
signs that improvements in Indigenous health indicators can be achieved when
services have sufficient resources to provide a sound base for primary health
care and where local communities take a strong role in developing and
delivering services. For example, at one
of the trials, the child immunisation rates have reached very high levels for
the first time. At another trial, preliminary data indicate a significant
increase in access by Aboriginal women to antenatal care services.[152]
4.20
Evidence to the Committee
during the inquiry, however, indicated some problems with the trials. The
Australian Medical Association (AMA) (NT Branch) argued that while in some
communities the trials are working well, including the Tiwi trial, there were
several problems ‘on the ground’ with these trials in relation to the availability
of doctors in Aboriginal communities and accountability in funding
arrangements. AMA (NT) stated that:
...the trials really will
not work unless there are more doctors on the ground in these areas...The second
thing is there is concern about the transparency of this paying out of funds
and where the money is actually going to and how it is being used by the
communities or the people who are the gatekeepers for these funds.[153]
4.21
AMA (NT) further stated that
the trials are ‘fine in terms of identifying unwell Aboriginal people, making
sure that they are followed up effectively and in getting the right
investigations done, but then it is actually treating these people and making
sure that you have done the groundwork, that you have worked out what is wrong with
them and you know what is needed to improve their quality of life. But actually
having the doctors on the ground to supervise that and to ensure that happens
is another problem’.[154]
4.22
The Northern Territory Branch
of the Australian Nursing Federation (ANF) also raised problems with
accountability. ANF (NT) noted that while the trials were ‘positive’ in that
they reflected a trend in Aboriginal communities of developing local control of
their own health services, the downside was a concern ‘about the sorts of
people that are attracted to the health boards that have been set up to run
those services’. It was argued that there was a need for more Commonwealth
scrutiny of the funds that are put into these programs.[155]
Effectiveness of the general trials
4.23
The interim evaluation report
of the general trials found that it was too early to conclude definitively that
the Coordinated Care Trials have achieved their objectives. The evaluation
report stated that the interim findings ‘cannot be seen as conclusive, but
rather should be used to give direction to future developments in coordinated
care’.[156] The report noted that the
complex nature of the trials and difficulties with ‘data flow, data quality and
data completeness, as well as by the diversity of trial populations and
processes’ made evaluation of the trials difficult.[157] The key findings of the interim
evaluation are outlined below.
Client health and well-being
4.24
The evaluation report stated
that the interim results of the trials on client health and well-being,
hospitalisation, re-admission and length of stay are inconclusive. Available
data indicate, however, that care coordination has not led to any significant
change in the health and well-being of the trial groups. The evaluation report
noted, however, that the data set is incomplete for some trials and that, while
the results are not statistically significant, trends suggest that some client
groups have experienced some improvements in their physical health status.[158]
4.25
A number of indicators of
health and well being were considered in the report, including hospitalisation
rates, re-admission rates within 28 days for the same cause, and length of stay
in hospital. The results showed that coordinated care had little or no effect
on these outcomes, with the exception of one trial that had lower hospital
re-admission rates.[159]
4.26
Regarding hospitalisation,
overall 25 per cent of clients had been hospitalised at least once over the
course of the trials, and this proportion was similar in the intervention and
control groups. The number of admissions that each person had was also similar
for the two groups. The proportion of re-admissions by trial varied
considerably, ranging from 6 to 16 per cent. Patients in the intervention group
of three trials had a statistically significant higher rate of hospital
re-admission than the control group. Only one trial had a statistically lower
rate of re-admission in the intervention group. After adjustments for age and
diagnosis-related group, these differences were no longer statistically significant,
with the exception of one trial that maintained a significantly lower rate of
re-admission in the intervention group. Regarding length of stay, while
individual trials did show some differences between intervention and control
groups, they were not statistically significant.[160]
4.27
Qualitative data were also
examined for evidence of the impact of care coordination on client health and
well-being. There were indications that some clients experienced an increased
sense of security as a result of having access to someone who could help them
to negotiate through the complexities of the health system. The perceptions of
moderate and high-risk clients were more positive than those of low-risk
clients, who tended to see care coordination as a hindrance rather than a help.
However, qualitative data were incomplete for some trials.[161]
Provider experiences
4.28
Providers include those
involved in the direct process of care coordination or in the delivery of
services.
4.29
In relation to GPs, not all
were willing to become involved in the trials. Their main concerns were the
additional administrative demands placed on them by the trials and a belief
that coordinated care would compromise their independence in treating their
patients. The GPs involved in the trials had different perceptions depending on
the model of care coordination used. GPs undertaking the role of care
coordinator had concerns about the time take to complete tasks associated with
coordinated care, the training required and the ‘time costs’ for any benefits
gained through the trials. GPs involved in care coordination where the tasks of
coordination were shared with others expressed some of these concerns, but were
generally more positive.[162]
4.30
Non-GP care coordinators
expressed concerns in relation to uneven workloads, their relationship with GPs
and the extent of their contribution to service coordination. Service providers
differed markedly in their perceptions of the trials. Some found that
coordination of care had freed them from case management, allowing them to focus
more on service provision, while others were concerned about increased
workloads and reduced resources.[163]
Substitution between services
4.31
An aim of the trials was to
promote further opportunities for appropriate substitution between acute and
sub-acute and community based services; community based services and
residential care; and a range of other community-based services.
4.32
The evaluation report stated
that the interpretation of service substitution varied across the trials. While
the majority of trials focussed on strategies to reduce hospital admissions,
the data do not indicate any effect on the rate of hospitalisation. Due to data
limitations it was not possible to establish whether any service substitution
had occurred.[164]
Range of services
4.33
The scope of pooling of
services can substantially influence the infrastructure costs of a trial. The
report noted that trials that pooled less widely than others, for example,
those that pooled only hospital, MBS and PBS have not demonstrated differences
in their ability to provide care within existing resources. The non-pooling of
residential care appears to have had an impact on a number of trials that have
anecdotal evidence of having delayed institutionalisation. The risk of cost
shifting by trials that pooled narrowly remains - however, there was no
evidence of this partly because the data were not available.[165]
Care coordination process
4.34
All trials demonstrated a
similar approach to the care coordination process which comprised assessment,
care planning, implementation, monitoring and review. How the various
components were put into operation varied according to the model of care
coordination within which they were placed. In all trials, GPs played a central
role, whether in the development of the care plans and/or implementation,
monitoring or review. Demand placed on GPs, both as a consequence of the trials
or external factors, restricted their capacity in some cases to be fully
involved in care coordination. Models in which GPs were supported in their
contribution to care coordination, through access to a care or service
coordinator, appeared to have been more satisfactory to all those involved in
the process.[166]
Financial outcomes
4.35
A ‘snapshot’ of the financial
status of each trial was made, and adjustments made for differences in
financial reporting and fund pool estimation. This analysis found diversity in
the way that trials allocated start-up and continuing costs, and also in the
way that the total costs of the trials were distinguished from running costs. A
comparison of trials’ total income with total expenditure, found that two
trials were significantly in deficit and one trial was slightly in deficit. The
report noted that, due to lack of data, conclusions about whether financial
decisions were appropriately made and key priorities chosen would need to be
considered in the final evaluation report.[167]
4.36
The report noted that there was
little evidence of coordinated care having an impact on the average cost or
distribution of services. For example, only one trial showed a statistically
significant reduction in the average cost of inpatient services. For a number
of trials, comparisons between the trial expenditure and the economic benchmark
(resources that would otherwise have been used), suggest that there are likely
to be gains made from coordinated care.[168]
Lessons from the trials
4.37
The evaluation report noted
that several key lessons emerged from the operation of the trials which are
outlined below:
-
coordinated care - funds pooling offers
potential advantages to facilitating care coordination for some, but not all
clients. This needs to be set against the considerable human and financial
resource costs associated with establishing and effectively managing the funds
pool. Improved targeting of people who need care coordination, and the
differentiation of care coordination approaches is also likely to be central in
maximising the value of coordinated care;
-
models of coordinated care - to be effective,
coordinated care requires a primary team approach, with GPs playing an important
and integral role. There needs to be a more systematic approach to coordinated
care in future trials, based on agreed eligibility criteria, better defined
target populations and standardised definitions of coordinated care and its
processes; and
-
role of GPs - given the pivotal role of GPs in
continuing care of patients with chronic conditions, the reasons why GPs choose
not to participate in the trials and the concerns expressed by participating
GPs need to be considered in the planning of future care coordination programs.[169]
Future directions
4.38
Some evidence suggested that
the coordinated care trials should be broadened in scope and extended in time
and coverage.[170] Professor Richardson
of the Centre for Health Program Evaluation (CHPE) suggested that the trials
could be broadened by extending the target population beyond persons with
complex chronic needs to the full population of a region. This would allow
preventive services to become a larger part of the model. But a longer time
frame would be required to test this type of model.[171]
4.39
Professor Hindle also argued
that it would be preferable to ‘run a demonstration project for an entire
community such the Hunter Valley or the ACT...it has to be a trial of the system
as it would operate in the real world and not where people can opt out, and so
on’.[172] NSW Health advised the
Committee that it is currently conducting a study into the feasibility of
introducing a funds pooling arrangement in two or three Area Health Services in
that State. Dr Picone from the Department stated that:
We think our area health services lend themselves more than in
some of the other states to allow this to happen because they have been running
for over a decade now...They are based on a population of people rather than on a
disease [model] because, if we go down the disease model, there is a chance of
reinforcing the lack of integration around the care of a human being that we
have got. The area health services have responsibility for the care of that
population and not just hospital care. Also, we have fairly sophisticated
funding arrangements.[173]
4.40
The Coordinated Care Trials
evaluation report also stated that extending the length of the trials would
increase the likelihood that effects such as reduced rates of hospitalisation
would be demonstrated within the time of the trial. The report also noted that
extending the trials would also reduce the average cost per client day and
improve the trials’ financial position, particularly for trials with
significant start-up costs.[174]
4.41
Professor Judith Dwyer representing
the Public Health Association of Australia argued that what was needed was a
‘move on from the second round of the coordinated care trials into some sort of
experimentation at the level of what kind of system you need to have in order
to deliver integrated or coordinated care, rather than simply looking at it at
the patient care level, which is what the coordinated care trials have done’.[175]
4.42
Professor Richardson suggested
that another option in relation to the trials would be to cover a more comprehensive
range of services and include, for instance, residential care, dental and
disability services. He argued that the reason for including residential care
is compelling because if care coordination reduces admission to residential
care facilities, the benefits of that should flow into the pool. The inclusion
of residential care would also increase the size of the pool, as many of the
chronically ill are also elderly. Extension to other areas is desirable, as the
aim is to break down program barriers and ensure access to care which is
appropriate to the health needs of the client group.[176]
Conclusion
4.43
The Committee notes the interim
evaluation reports on the coordinated care projects that have been published
and the various suggestions made to overcome the problems identified in the
initial evaluations. A full picture of the value of coordinated care and the
role it may play for specific groups or wider communities has not been possible
due to limitations in the data available from these studies. The Committee is
disappointed that the data from these initial evaluations is not more complete
and that conclusions about the effectiveness of the trials could not be drawn.
It is hoped that a more complete assessment of the trials will emerge when the
final studies are complete.
4.44
The Committee commends the
Government for committing to a second round of Coordinated Care Trials and
urges that work continue on developing the most suitable form of coordinated
care for Australian circumstances within the framework of Medicare. The
Committee believes that better data should be available and collected with the
additional trials to allow informed conclusions about the efficacy of these
trials to be drawn.
Recommendation 19: That Health Ministers ensure that the
additional Coordinated Care Trials be designed to include adequate and
appropriate data for collection and analysis to enable informed conclusions
about the effectiveness of these trials.