Chapter 5 - The expert committees and ministerial council
5.1
This chapter discusses the
functions and powers of the Gene Technology Technical Advisory Committee, the
Gene Technology Community Consultative Group and the Gene Technology Ethics
Committee; and the role and membership of the proposed Ministerial Council. The
chapter also discusses the procedures for review of decisions and, in
particular, the rights of third-parties to seek review of decisions of the
Regulator.
5.2
The Bill provides for the
establishment of a Gene Technology Technical Advisory Committee, a Gene
Technology Community Consultative Group and a Gene Technology Ethics Committee
to provide expert advice to the Regulator and the Ministerial Council
overseeing the national legislative scheme.
Figure 2: Proposed
regulatory structure under the Gene
Technology Bill
Gene Technology Community Consultative Group
Gene Technology Technical
Advisory Committee
Gene Technology Regulator
Ministerial
Council
Gene Technology Ethics
Committee
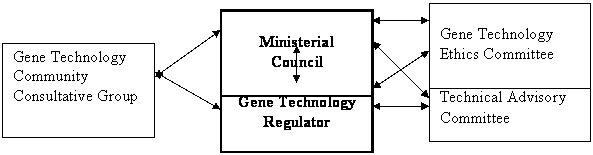
Source:
Submission No.77, p.106 (IOGTR).
5.3
The following sections discuss
the functions and powers of the three proposed advisory committees.
Gene Technology
Technical Advisory Committee
5.4
The function of this committee
is to provide scientific and technical advice, at the request of the Regulator,
or of the Ministerial Council, on a range of matters including:
-
gene technology, genetically modified organisms
(GMOs) and genetically modified (GM) products;
-
applications made under the Bill;
-
the biosafety aspects of gene technology; and
-
the need for policy principles, policy
guidelines, codes of practice and technical and procedural guidelines in
relation to GMOs and GM products and the content of such principles, guidelines
and codes.[421]
5.5
The committee is to comprise up
to 20 part-time members appointed by the Minister. The Minister appoints the
chair of the committee, but must not appoint a member to chair the committee
unless a majority of the States and Territories agree to the appointment.[422]
5.6
Prior to appointing any members
the Minister must consult a range of stakeholders including the States; the
Regulator; scientific, consumer, health, environmental and industry groups; and
any Ministers considered appropriate. The areas of expertise to be reflected on
the committee include molecular biology; ecology; plant, animal or human
genetics; virology; entomology; agricultural systems; biosafety engineering;
public health; and occupational health and safety; risk assessment; clinical
medicine; biochemistry; pharmacology; plant or animal pathology; microbiology;
and animal biology. The committee members are subject to conflict of interest
and disclosure of interest requirements, which are outlined later in this
chapter. The Minister must also appoint a layperson as a member of the
committee. The Minister may also appoint one or more ‘expert advisers’ to the
committee. These advisers may be appointed on an ad hoc or continuing basis and
will be expected to supplement the expertise of the committee where this is
necessary in relation to the consideration of particular applications.[423]
Role of the committee
5.7
Several submissions commented
on the limited powers of the committee. Submissions noted that the committee is
only able to act on the request of the Regulator or the Ministerial Council. It
was argued that the committee should be required to provide advice on all
aspects of dealings that may pose a significant risk to human health or the
environment.[424] The Australian
Conservation Foundation (ACF) argued that the Bill should be amended to ensure
that this committee, and the other two advisory committees, are consulted by
the Regulator in consideration of all licence applications.[425]
5.8
The Interim Office of the Gene
Technology Regulator (IOGTR) noted, however, that the committee ‘will consider
individual applications and provide scientific advice on the possible risks
posed by the application to public health and safety and to the environment’.[426] The Regulator will take this advice
into account, along with advice received from public submissions, other
Commonwealth agencies and the Environment Minister, in preparing risk
assessment and risk management plans.[427]
The committee has, however, no power to initiate the provision of advice and/or
information on a dealing, GMO or GM product. In addition, in most matters the
Regulator is under no obligation to seek the advice of the committee on any
matter.
5.9
The IOGTR stated that in most
overseas regulatory regimes ultimate responsibility for the scientific
assessment of risk, and the approval of any applications, lies with an
independent decision-maker like the GTR, rather than a committee of experts.
Many regulators do, however, seek advice from an expert committee. For example,
in Germany, the Federal Ministry of Health seeks advice from the Advisory
Committee for Biological Safety. In Canada, the Federal Minister for the
Environment seeks advice from the National Advisory Council on the management
of toxic substances.[428]
5.10
The Committee considers that
the proposed functions of the Gene Technology Technical Advisory Committee as
outlined in the Bill are adequate. The Committee believes that the scientific
committee in providing scientific and technical advice, at the request of the
Regulator, or the Ministerial Council, on a range of matters, including licence
applications, will play an important role in the regulatory system.
Composition of the committee
5.11
Several submissions commented
on the narrow proposed range of experts on the committee. Submissions argued
that the membership of the committee will be dominated by gene technologists.[429] ACF GeneEthics Network argued that
‘these people are not independent or impartial. Environmental and other
relevant technical and scientific experts must be included. Without a broad
range of expertise, this committee will be like GMAC, not a genuinely rounded
expert body’.[430]
5.12
One submission argued that the
membership of the committee should be reviewed to include experts in the fields
of nutrition, agriculture, animal husbandry, veterinary science and
environmental science.[431] Another
submission argued for the addition of economists, ethicists and trade experts
to ensure that the impact of GMOs is considered in the ‘broadest possible way’.[432]
5.13
It was argued that it was
important that this committee not be the captive of ‘vested interests’ as it is
the only committee to view, and advise on, applications for permits for GM
work.[433]
5.14
One submission also questioned
the effectiveness of having only one layperson in a committee of 20 experts and
suggested that the person would be ‘overwhelmed’.[434] The IOGTR stated the inclusion of a
layperson on the committee was supported by many people during consultations
and reflects the current GMAC practice.[435]
5.15
Several submissions also argued
that there needs to be cross-membership of the committees with a representative
of the community consultative group and the ethics committee on the scientific
committee.[436] While the IOGTR stated
‘there is actually a requirement in the legislation that there be
cross-membership between all three committees’[437], the Committee notes that the
proposed legislation only makes it a requirement that:
-
the Minister must appoint a layperson as a
member of the Gene Technology Technical Advisory Committee;
-
the Minister must ensure that the Gene
Technology Community Consultative Group includes the following members:
-
a person who is a member of Gene Technology
Technical Advisory Committee; and
-
a person who is a member of the Gene Technology
Ethics Committee; and
-
the Minister must ensure that the Gene
Technology Ethics Committee include a member of the Gene Technology Technical
Advisory Committee.
Recommendation
The Committee RECOMMENDS that the Bill be amended to require
that the Gene Technology Technical Advisory Committee include a member of the
Gene Technology Community Consultative Group and a member of the Gene
Technology Ethics Committee, and preferably that that person should be the
Chair of their respective committee.
5.16
The Committee believes that the
Gene Technology Technical Advisory Committee should essentially be comprised of
members who are able to provide scientific and technical advice to the
Regulator and the Ministerial Council but should comprise members with a broad
range of scientific and related expertise, including environmental and other
relevant technical and scientific experts, and represent a diverse range of
scientific views.
Recommendation
The Committee RECOMMENDS that the Bill be amended to require
the Minister, in appointing members of the Gene Technology Technical Advisory
Committee, appoint members representative of a range of scientific disciplines
and a diverse and broad range of scientific views.
Disclosure of interests
5.17
Several groups and individuals
argued that there was a need to ensure committee members are subject to strict
disclosure of interest provisions. Submissions argued that all members of the
committee should be under a statutory obligation to disclose all interests in
the development and commercialisation of gene technology. Further, members
should be obliged to perform their duties in an independent manner and must be
required to excuse themselves from participating in matters where there is a
potential for a conflict of interest.[438]
5.18
The draft Regulations, tabled
on 25 August 2000, detail the conflict of interest and disclosure of interest
requirements. The Committee regrets that the draft Regulations were available
so late during the Committee’s inquiry. This did not provide witnesses with the
opportunity to comment on the adequacy or otherwise of the Regulations during
the Committee’s hearings into the Bill.
5.19
Under the draft Regulations,
committee members, and any expert advisers, will be required to:
-
make a declaration setting out all direct or
indirect interests, financial or otherwise, that a person is aware that he or
she has in any matter of a kind to be considered at a meeting of the committee,
before being appointed; and
-
a member who is aware that he or she has a
direct or indirect interest, pecuniary or otherwise, in a matter being
considered, or about to be considered, at a meeting of the committee must
disclose the nature of the interest at, or before, the meeting of the
committee. Disclosure must include interests of the member, of the member’s
spouse, of parents of the member and of the spouse, of the children of the
member and of any other children of the member’s spouse, that provide, or could
provide, a substantial source of income or a substantial asset; and could be
perceived to represent a possible conflict of interest.
The disclosure must be recorded in the minutes of the
meeting and the member must not be present during any deliberation of the
committee about the matter; nor must he or she take part in any decision of the
committee about that matter.[439]
5.20
The Committee believes that
committee members should be subject to strict disclosure of interest
provisions. The Committee believes that the proposed disclosure of interest
provisions are adequate and appear to meet the concerns expressed in evidence
regarding the need for stringent disclosure provisions.
Terms of appointment
5.21
Submissions also argued that
there should be fixed and specified terms of tenure for committee members as
the positions are potentially very influential given the commercial aspects of
the work.[440]
5.22
The Regulations specify that
members of the committee are to be appointed for a period of three years, or a
lesser period specified in writing. The IOGTR stated that the period of three
years was selected because it balances continuity of membership with the need
to ensure that membership does not become static. It is anticipated that
changes in membership will be staggered to ensure there is not a complete
turnover of committee members, with resulting loss of accumulated knowledge,
every three years.[441]
Gene Technology Community Consultative Group
5.23
The function of the
Consultative Group is to provide advice, at the request of the Regulator or the
Ministerial Council, on matters of general concern in relation to GMOs, and on
the need for, and content of, policy principles, guidelines, codes of practice
and technical and procedural guidelines in relation to GMOs and GM products.[442]
5.24
The IOGTR stated that it is
expected that the committee will fulfil this role by ensuring that the
Regulator and the Ministerial Council are kept in touch with community views.
The Group may do this by:
-
providing advice on how it thinks community
consultations might most effectively be undertaken;
-
providing advice on draft codes of practice
developed by the Regulator;
-
suggesting that certain policy principles be
developed; and
-
raising issues of ethical concern that they wish
to be examined by the Gene Technology Ethics Committee.[443]
5.25
The Minister is to appoint up
to 12 members of the Consultative Group on a part-time basis. Prior to appointing
the members the Minister must consult the Regulator; the States; such
scientific, consumer, health, environmental and industry groups considered
appropriate as well as other Ministers considered appropriate. Appointees to
the committee must have skills or experience pertaining to gene technology in
one or more of the following areas - environmental issues; consumer issues; the
impact of gene technology on the community; issues relevant to the
biotechnology industry; issues relevant to gene technology research; public
health issues; issues relevant to primary production; and issues relevant to
local government.
5.26
The Consultative Group must include a person
who is a member of the Gene Technology Technical Advisory Committee (to provide
scientific assistance to the Group); and a person who is a member of the Gene
Technology Ethics Committee (to assist with advice on ethics issues).[444] The Minister appoints the chair of
the committee but may not appoint a chair unless a majority of the States and
Territories agree to the appointment.[445]
5.27
The IOGTR stated that it is not
intended that the appointees to the Consultative Group be scientific experts in
gene technology, rather it is expected that they will be able to speak to
certain issues that are relevant to gene technology, such as environmental or
consumer issues.[446]
Role of the Consultative Group
5.28
Several groups argued that the
role of the Group is too limited and that it should be consulted in relation to
all licence applications considered by the Regulator.[447] Submissions noted that the role is
only confined to providing advice at the request of the Regulator or the
Ministerial Council.[448] GE-Free
Tasmania stated that the consultative group needed to have an expanded role so
that it can ‘take on a more pro-active role, and be able to initiate the
advisory process where they feel it is appropriate’.[449] The Australian GeneEthics Network
(AGN) concurred with an expanded role, suggesting that the Group ‘should
actively hold roundtables and seek public support for the development of strong
policy on broad categories of GE work’.[450]
5.29
As noted previously, only the
Gene Technology Technical Advisory Committee will be directly involved in
providing advice on GMO licences and other applications (clause 101). The
Community Consultative Group (and the Ethics Committee) will be consulted only
in relation to general principles or guidelines, not in relation to specific
decisions.[451]
5.30
It was argued that the
Consultative Group’s brief should be broadened and its decisions should have
the same weight and standing as GTAC decisions, so that it wins the confidence
of the public. As the Organic Federation of Australia (OFA) stated: ‘to limit
this group to providing advice on policy only does not do justice to concerns
in the community about the way regulation is made, and the desire for the
community to move beyond the simplistic notion that decisions must be based on
science and logic’.[452]
5.31
The IOGTR stated that
individual applications for licences are not being referred to the Community
Consultative Group:
...on the basis that, as a result of other mechanisms incorporated
into the proposed scheme, there is already extensive opportunity for community
input on individual applications...Given this high level of community
involvement in decision making (unrivalled in most existing regulatory
schemes), the Commonwealth States and Territories did not consider that it was
necessary to incur additional expense and resources by duplicating the
consultation process and also tasking the GTCCG with examining individual
applications.[453]
5.32
The Interim Office also noted
that the establishment of a statutory community consultative group advising on
matters of policy is itself fairly unique in both the Australian regulatory
environment and internationally. The IOGTR stated that most of the existing
Australian schemes for regulation of GM products do not have statutory
established community committees. The Australian Quarantine and Inspection
Service (AQIS), for example, does not have a community advisory committee to
assist in its assessment of imports for quarantine risks, although it does
involve the community through its consultation process. The Therapeutic Goods
Administration (TGA) is, however, proposing to establish, through
administrative arrangements, a Consumer Health Forum.[454]
5.33
The Interim Office further
stated that internationally most countries have not established a community
consultative group under legislation and where community groups are utilised
they provide advice on policy rather than on individual applications. In the
case of the United Kingdom, the Agriculture and Environment Biotechnology
Commission has been established to advise the Government on GM foods. While the
Commission has strong community representation it will not comment on
individual applications.[455]
5.34
Several submissions also argued
that meetings of the Community Consultative Group and the technical committee
should be held in public, exempting commercial in confidence items, and that
they report all proceedings on the Internet. It was also argued that meetings
should be convened around Australia to
ensure a range of views are heard and evidence is received from a wide-range of
interested citizens [456] OFA argued that this would ‘ensure
there is true transparency in monitoring the workings of these advisory
committees’.[457] One submission argued
that a local government representative should be a mandatory appointment to the
committee.[458]
Terms of appointment/disclosure of
interests
5.35
Several groups argued that
there needs to be fixed and specified terms of tenure and strict provisions to
ensure that members of the consultative group have no conflict of interest
relating to their functions.[459]
5.36
The draft Regulations (Part 5)
mirror the provisions that apply in relation to the conditions of appointment
for the scientific committee. The Regulations provide that the members of the
committee will be appointed for three year terms, and must abide by strict
disclosure of interest provisions (as discussed in the previous section).
Conclusion
5.37
The Committee believes that Gene
Technology Community Consultative Group should have a broader role than merely
limited to matters of general concern in relation to GMOs, and on the need for
policy principles, guidelines and codes of practice.
5.38
The Committee believes that
there needs to be greater community input into the decision-making processes in
relation to licence applications especially in light of the potential impact of
gene technology on human health and on the environment and the need for
effective community involvement in the regulatory processes. The Committee
therefore considers that the Bill should provide that the Community
Consultative Group provide advice on individual licence applications.
Recommendation
The Committee RECOMMENDS that the Bill be amended to require
that the Gene Technology Community Consultative Group provide advice on
individual licence applications made under the Bill.
Gene Technology Ethics Committee
5.39
The function of the committee
is to provide advice, at the request of the Regulator or of the Ministerial
Council, on ethical issues relating to gene technology; the development of
codes of practice in relation to ethics in respect of conducting dealings with
GMOs; and the development of policy principles in relation to dealings with
GMOs that should not be conducted for ethical reasons.[460]
5.40
As in the case of the community consultative
group, before appointing members to the committee, the Minister must consult
the States, through the Ministerial Council, the Regulator, appropriate
scientific, consumer, health, environmental and industry groups and appropriate
Commonwealth Ministers. The Minister must ensure that the composition of the
committee includes a member of the Technology Technical Advisory Committee, as
well as a member of the Australian Health Ethics Committee with expertise in
medical research.[461] The Minister
appoints the chair of the committee, but may not appoint a chair without a
majority of States and Territories agreeing to the appointment. The Minister
may appoint one or more expert advisers to the committee. These advisers may be
appointed on an ad hoc or continuing basis and will be expected to supplement
the expertise of the committee where this is necessary in relation to the
consideration of particular matters.[462]
5.41
There was general support for
the establishment of the ethics committee. Heritage Seed Curators Australia
(HSCA) stated ‘we believe that the moral and ethical dimensions to gene
technology are extremely important, However, this aspect goes largely ignored
in the general debate on GE [genetic engineering]. We trust that the creation
of this committee will bring this aspect of GE more to the fore in future’.[463] CSIRO also welcomed the proposed
establishment of the committee but ‘attaches urgency to its formation and
productive output, particularly the provision of ethical codes with a strong
focus on the practical means by which their tenets are to be applied’.[464]
5.42
The IOGTR stated that the
involvement of an independent ethics advisory committee in the regulation of
gene technology places Australia ahead of similar regulatory schemes overseas.
For example, no statutory ethics committee is involved in providing policy
guidance in the United States, New Zealand, Japan or Canada. The IOGTR also
noted that neither AQIS, TGA, the National Industrial Chemicals Notification
and Assessment Scheme (NICNAS), the Australia New Zealand Food Authority
(ANZFA), or the National Registration Authority (NRA) have established expert
ethics committees.[465]
Role of the committee
5.43
Several groups argued that the role of the
committee, as with the community consultative group, is too limited and that it
should be consulted in relation to all licence applications considered by the
Regulator.[466] Submissions noted that
the role is only confined to providing advice at the request of the Regulator
or the Ministerial Council.[467] HSCA
also suggested that the committee’s meetings should be public to enhance
consumer confidence in the regulatory process.[468]
5.44
As noted previously, under the
Bill as it stands, only the scientific committee will be directly involved in
providing advice on GMO licences. The ethics committee and the community
consultative group will be consulted only in relation to general principles or
guidelines, not in relation to specific decisions. The ethics committee, along
with the other two committees, may be consulted in relation to the need for,
and content of, policy principles guiding the ethical decisions of the
Regulator, and codes of practice applicable generally to dealings with GMOs.[469]
5.45
Evidence emphasised the need
for an overlap in the membership of the committees. Dr Roush stated that ‘if
you really want ethics to infuse the whole debate, why not thoroughly integrate
the so-called ethics committee, or the ethicists that are involved, in both the
technical committee and the community committee. Why have a separate entity? If
anything it reinforces the public view that ethics is over here and scientists
are over here and the twain never meet.[470]
Terms of appointment/disclosure of
interests
5.46
Several groups argued that
there needs to be fixed and specified terms of tenure and strict provisions to
ensure that members of the committee have no conflict of interest relating to
their functions.[471]
5.47
The draft Regulations (Part 6)
mirror the provisions that apply in relation to the conditions of appointment
for the scientific committee and the community consultative group. The
Regulations provide that the members of the committee will be appointed for
three year terms, and must abide by strict disclosure of interest provisions (as
discussed in the previous sections).
Conclusion
5.48
The Committee believes that the
Gene Technology Ethics Committee should have a broader role than that envisaged
in the Bill and that the moral and ethical dimensions in relation to gene
technology should be considered in relation to licence applications.
5.49
The Committee therefore considers that the
Bill should provide that the Regulator may, if he or she deems it necessary,
refer individual licence applications to the Gene Technology Ethics Committee
for advice.
Recommendation
The Committee RECOMMENDS that the Bill be amended to provide
that the Regulator may, if he or she deems it necessary, refer individual
licence applications to the Gene Technology Ethics Committee for advice.
The Ministerial Council
5.50
The Bill provides that a
Ministerial Council comprising Ministers from the Commonwealth and each State
and Territory will be established, under an Intergovernmental Agreement on Gene
Technology (IGA), to provide broad oversight of the regulatory framework and guidance
on matters of policy that underpin the legislation. The Ministerial Council
will be responsible for:
-
undertaking general oversight of the
implementation of the scheme and considering and agreeing any proposed changes
to the national scheme;
-
issuing ‘policy principles,’ ‘policy guidelines’
and ‘codes of practice’ to underpin the regulatory system (see below);
-
seeking advice from each of the statutory
committees;
-
ensuring coordination with other Ministerial
Councils on matters relating to gene technology; and
-
advising on the appointment and termination of
the Regulator and the Chairpersons of the three committees to be established
(see above).[472]
5.51
‘Policy principles’ are
disallowable instruments and therefore are subject to review by the Parliament.
Policy principles deal with ethical issues relating to GMOs or other matters
prescribed by regulations (clause 21), and are issued by the Ministerial
Council after consultation with relevant Commonwealth, State, industry and
community organisations, including the three advisory committees (clause 22).
The Regulator must not issue a GMO licence that is inconsistent with a policy
principle (clause 57).[473]
5.52
‘Policy guidelines’ are issued
by the Ministerial Council, and may deal with matters relevant to the
Regulator’s functions. They will be guidance notes to the Regulator, and will
not be prohibitive or akin to a direction, but will be advisory. The Regulator
must have regard to policy guidelines in deciding whether or not to issue a GMO
licence (clause 56), but is not bound to follow them. Unlike policy principles,
policy guidelines are not required to be made in consultation with anyone,
although the Ministerial Council may choose to consult. Policy guidelines are
not disallowable instruments (clause 23), and therefore are not subject to
Parliamentary scrutiny.[474]
5.53
‘Codes of practice’, with which
GMO licence-holders may be required to comply, are developed by the Regulator
and issued by the Ministerial Council
after extensive consultation with each of the committees, relevant
Commonwealth and State agencies and industry and consumer groups. They will be
disallowable instruments (clause 24), and therefore subject to Parliamentary
scrutiny. The Regulator may apply a requirement that a code of practice be
complied with as a condition of licence.[475]
Policy guidelines
5.54
Submissions argued that the
Bill should be amended to ensure that the three advisory committees are
consulted by the Ministerial Council when issuing policy guidelines.[476] As discussed above, unlike policy
principles and codes of practice, policy guidelines are not required to be made
in consultation with anyone, although the Ministerial Council may choose to
consult.
Recommendation
The Committee RECOMMENDS that the Gene Technology Technical
Advisory Committee, the Gene Technology Community Consultative Group and the
Gene Technology Ethics Committee be consulted by the Ministerial Council when
issuing policy guidelines.
Veto on licence applications
5.55
Some groups proposed that the
Ministerial Council should have the power of veto on licences approved by the
Regulator or be able to strengthen or include new conditions on a licence
granted.[477]
5.56
The IOGTR stated that
Commonwealth and State Governments considered that Ministerial direction or a
power of veto on individual decisions by the Regulator would undermine the
independence of the Regulator and cast aspersions on the Regulator’s integrity
and freedom from political processes.[478]
The Australian Food and Grocery Council (AFGC) also stated that it was
important that the Council not be involved in the day-to-day operation and
decision making of the Regulator.[479]
5.57
The IOGTR noted that this
approach is consistent with other regulatory systems in Australia. For example,
in the case of therapeutic goods, the Minister sets policy and standards while
the delegate of the Secretary of the Department of Health and Aged Care (DHAC)
decides on individual applications for drug approval. Under the system of food
regulation, the Australia New Zealand Food Standards Council has the power of
veto over food standards, but does not rule on the application of these
standards to individual cases - which is the responsibility of ANZFA.[480]
5.58
The Committee does not consider
that the Ministerial Council should have the power of veto on licences approved
by the Regulator. The Committee believes that Ministerial direction, or a power
of veto on individual decisions would undermine the independence of the
Regulator.
Membership of the Council
5.59
It is proposed that the
Ministerial Council be comprised of one Minister representing each
participating jurisdiction. The IOGTR stated that the Minister representing
each jurisdiction will be determined by the governments of each jurisdiction.
The Commonwealth will be represented by the Minister for Health and Aged Care.[481]
5.60
Consumer and other groups
argued that the Ministerial Council should comprise either Health and/or
Environment Ministers.[482] OFA,
arguing for the inclusion of Health and Environment Ministers on the Council,
stated that this was necessary ‘to give public confidence in the protection of
health and safety and the environment’.[483]
The National Farmers’ Federation (NFF) argued that the Council should include
representation from the Agriculture Minister.[484]
NSW Farmers’ Association argued for representation from Agriculture and Foreign
Affairs and Trade Ministers, noting that it was important that there is
representation of affected parties such as the agricultural industry on the
Ministerial Council.[485]
5.61
AFGC argued that the Council
should include Ministers from a range of portfolios so that the expertise
resident in the different areas relevant to gene technology can be brought into
the deliberations of the Council. AFGC argued that gene technology impacts on a
wide range of portfolios including Health, Environment, Agriculture/Primary
Industry, Science and Technology and Trade and Commerce.[486]
5.62
The Committee sought
clarification from the IOGTR as to whether Ministers on the Council will
comprise the same members or whether a State may elect to send different
Ministers to each subsequent meeting of the Council.
5.63
The IOGTR advised the Committee
that:
The expectation that will underpin the intergovernmental
agreement is that a consistent minister attends. If it is the agriculture,
health, environment or whatever minister, it is envisaged that they
consistently attend. There will certainly be flexibility to change the
minister, particularly when the legislation may move portfolios within
individual jurisdictions.[487]
5.64
DHAC advised the Committee that
the Intergovernmental Agreement (IGA) that established ANZFA does not specify
that the Health Minister will be the representative on the Council -‘the
practice of state governments has been to send the health minister, but it is
not enshrined as the health minister [in the IGA]’.[488]
5.65
The Committee considers that
the Ministerial Council should comprise one Minister representing each
participating jurisdiction. The Committee considers that given that gene
technology impacts on a wide range of portfolios it is important that the
Council should be comprised of Ministers, who, while representing a specific
portfolio, have an understanding of broader issues relevant to gene technology
and be able to bring a multi-faceted approach to the Council’s deliberations.
Rights of third-parties to seek review of decisions
5.66
Several groups argued that
third parties should have the right to seek review of a decision by the
Regulator.[489]
Review of decisions
5.67
The Bill provides the following procedures for
the review of decisions.
Division 2 of Part 12 of the Bill provides that certain
persons may seek internal review of decisions made under the legislation.
Essentially, those people include:
- licence applicants and licence holders;
- applicants for certification and holders of certification
(for example, universities or companies who have sought certification to a
certain containment level of the facilities owned or operated by them);[490] and
- applicants for accreditation and holders of accreditation
(for example, universities and institutions who have sought accreditation,
recognising that they have established and maintained an Institutional
Biosafety Committee within their institution).
5.68
If the relevant decision has
been made by a delegate of the GTR (for example, one of the senior staff
members of the GTR) the person seeking a review of the decision would have to
apply to the GTR for an initial review of the decision. The GTR would look closely at the delegate’s
decision and would substitute his/her decision where appropriate.[491]
-
Review by
the Administrative Appeals Tribunal
5.69
If the GTR made the decision
personally, or if a person has sought review by the GTR and seeks further
review of the decision, those people with standing (detailed above in relation
to internal review) may make an application for further review to the
Administrative Appeals Tribunal (AAT).[492]
-
Review by
the Federal Court under the Administrative Decisions (Judical Review) Act 1977
5.70
The Bill, however, does not
include any explicit provisions about who may apply to the Federal Court under
the Administrative Decisions (Judicial
Review) Act 1977 - AD(JR) Act. The IOGTR explained that this is because a
person may automatically seek review of a decision by the Federal Court
provided the person can meet the Court’s criteria for determining whether the
person is ‘aggrieved’ by a decision made under the gene technology legislation.
Therefore, there is no need, or requirement, for the capacity for review under
the AD (JR) Act to be referenced in the Bill.[493]
5.71
Any person wishing to have a
decision reviewed by the Federal Court under the AD (JR) Act must establish
‘standing’ or a ‘special interest’ as required by the Federal Court. While this
is judged on a case-by-case basis, the general position is that an applicant
must be able to show an interest above and beyond that of ordinary members of
the public.
5.72
For example, an organisation
that has as part of its constitution or terms of reference, a reference to gene
technology (such as the GeneEthics Network), would be likely to be able to
establish standing to seek review under the AD (JR) Act. Similarly, an organic
farmer whose property adjoined the property of a farmer growing GM crops would
be likely to be able to establish a ‘special interest’ in the relevant decision
of the GTR.[494]
5.73
The IOGTR stated that The Environment Protection Biodiversity
Conservation Act 1999 specifically provides that certain individuals are
taken to be aggrieved by a decision for the purposes of seeking review by the
Federal Court. For example, an
individual who has, in the two years immediately before the decision is made,
been engaged in a series of activities in Australia for protection,
conservation or research into the environment. This position essentially
reflects current ‘standing’ arrangements under the Federal Court. As such, it was
not considered necessary to specifically replicate this in the Bill.[495]
Views on rights of third parties to
seek review
5.74
As noted above, several
organisations argued that third parties should have the right to seek review of
a decision by the Regulator.[496] The
Australian Centre for Environmental Law (ACEL) argued that the Bill:
...unfairly discriminates against third parties wishing to appeal
the grant of licences and incorporates limited standing provisions reminiscent
of the 19th century. It certainly does not represent regulatory
“best practice” in the nascent 21st century. The right to appeal is
limited and exercisable only by applicants for licences. For members of the
public that comprise third parties, this limitation is clearly discriminatory,
against principles of natural justice, and against the public interest.[497]
5.75
ACEL agued that the Centre’s
review of environmental standing provisions around the world ‘establishes that
the “best practice” trend is towards open standing. Indeed, even the
Commonwealth’s most recent piece of environmental legislation, the EPBC Act,
creates limited open standing for any individual or organisation (whether
incorporated or not) that has been involved in conservation or environmental
issues over the previous two years’.[498]
5.76
GE-Free Tasmania acknowledged
that the provision of third party appeal provisions has the potential to place
strain on the resources of the Regulator and the AAT. Accordingly, the group
argued that it is appropriate that a limit be placed on the persons who should
have the right to appeal and that this be limited to third parties who have an
ongoing involvement in the GE debate and who seek to represent a significant
social interest or concern.[499]
5.77
Industry and primary producer
organisations did not support the need to make provision for third parties to
appeal the decisions of the Regulator.[500]
Industry groups stated that the Bill has extensive provisions which require the
Regulator to seek comment and have regard to that comment from the public on
risk assessment, risk management and licensing decisions (Division 2 of the
Bill).[501] Avcare Ltd commented that:
Licensing decisions and other actions of the Regulator are open
to interlocutory injunctions if a person or community group believes that an
inappropriate decision has been made. It is important that the Bill does not
facilitate vexatious appeals, with all the delays these involve.[502]
5.78
Meat and Livestock Australia
(MLA) stated that the proposed processes for review of decisions (Part 12,
Division 2) recognise the rights of both applicants and other stakeholders.
Applicants and holders of certification and/or accreditation have the
opportunity of review and appeal through the AAT - ‘which is an appropriate
impartial mechanism’.[503] MLA also
noted that the Regulator has the discretionary capacity to, at any time, vary,
suspend or cancel a licence, and to review decisions relating to exemptions
from the need for a licence and notifiable low risk dealings - ‘there are
adequate opportunities for third parties to raise objections relating to
decisions, with the Regulator having extensive discretionary power to act on
those objections if there are sufficient grounds’.[504]
5.79
The IOGTR stated that taking
into account the open assessment processes described under the Bill, States,
Territories and the Commonwealth considered that it would be appropriate to
limit the right of review to the AAT to people immediately affected by a
decision (such as licence applicants and licence holders). The States and
Territories reached this conclusion because:
-
the decisions of the Regulator are the product
of extensive consultation processes that would be time consuming and costly to
repeat on review;
-
the details of all decisions are made publicly
available on a database of decisions;
-
the Bill is drafted to carefully define the
people who are able to seek review of decisions. Under a number of other legislative schemes,
the class of people who are able to seek review of decisions is not quite so
clearly defined. This gives rise to a great deal of uncertainty, not only for
the regulator (who must make a decision on whether a complainant has a right of
review) but also for the general public, who have to seek interpretation from
the AAT as to whether they can seek review of a particular decision;
-
the review mechanisms are consistent with
similar legislation, including the Australia
New Zealand Food Authority Act 1991
(Cth). Section 63 of this Act restricts applications for AAT review of a
decision to reject an application to the applicant. There is no right of review over the decision
to accept an application, which would in turn lead to a change in the relevant
Standard;
-
limiting AAT review to people immediately
affected by a decision reduces the capacity for vexatious review of decisions.
Without this capacity, certainty of decision-making would be reduced as licence
holders, as well as holders of certifications and accreditations, could not
truly rely on the decision of the Regulator until all possible avenues of
review had been exhausted; and
-
limiting rights of review to the AAT is
consistent with Commonwealth policy. The approach adopted by the States,
Territories and the Commonwealth, as reflected in the Bill, has been considered
in detail by the Attorney-General’s Department, which advised that the approach
adopted is appropriate given the extensive consultation processes described in
the Bill.[505]
Conclusion
5.80
The Committee considers that
third parties should have the right to seek review of a decision by the
Regulator. The Committee believes that the Bill as it stands unfairly
discriminates against third parties wishing to appeal the grant of licences and
as such is discriminatory, against principles of natural justice, and against
the public interest and will undermine public confidence in the system.
Recommendation
The Committee RECOMMENDS that the Bill be amended to provide
for the right of third parties to apply for review of a decision of the
Regulator.