Chapter 2 - Background on gene technology
Introduction
2.1
The focus of the Committee’s
inquiry was to examine the proposed regulatory system for genetically modified
organisms (GMOs) as set out in the Gene Technology Bill 2000.
Understanding what is involved in gene technology is important when considering
the consequences of the products of this technology, and the adequacy of the
regulatory arrangements that have been formulated to ensure the protection of
the community and our environment.
2.2
This chapter aims to provide
sufficient information for people to understand gene technology, without
purporting to provide a detailed scientific explanation of the concepts and
processes associated with gene technology. The chapter also highlights some of
the concerns raised in evidence about the way the Bill defines genetically
modified organisms, and the risks and benefits associated with gene technology.
What is gene technology?
2.3
The principle of altering
various organisms is not new-for centuries, a range of techniques have been
used to alter the properties of plants and animals through selective breeding
or plant grafting. Today, gene technology has greatly increased the number of
plant and animal traits that can be manipulated and, significantly, transferred
across the species barrier.
2.4
Gene technology, sometimes also
referred to as biotechnology[11], has
been used to describe techniques involving the genetic modification of
organisms. Gene technology refers to ‘the transfer of DNA between living cells
to produce a certain outcome’.[12] Gene
technology has also been described as the field of research that uses ‘gene
transfer techniques to produce recombinant proteins and genetically modified
organisms’.
2.5
The Gene Technology Bill 2000
defines gene technology as ‘any technique for the modification of genes or
other genetic material’. The Bill defines a genetically modified organism (GMO)
as:
-
an organism (any biological entity that is
viable, capable of reproduction or capable of transferring genetic material)
that has been modified by gene technology; or
-
an organism that has inherited particular traits
from an organism (the initial organism), being traits that occurred in the
initial organism because of gene technology; or
-
anything declared by the regulations to be a
genetically modified organism, or that belongs to a class of things declared by
the regulations to be genetically modified organisms.
2.6
The use of the term GMO to
describe a genetically modified organism is often used interchangeably with the
expression GEO or genetically ‘engineered’ organism, although some may claim
that genetically modified is not an adequate description where recombinant DNA
techniques have been used. Organisms that have been genetically manipulated
have also been described as having been ‘genetically improved (GI)’. This
report uses the term GMO to refer to organisms that have undergone genetic
modification, except where the report has quoted directly from evidence or
submissions which use an alternative expression.
2.7
The term transgenic is often
broadly used to mean genetically modified. A more generally recognised
understanding of the term is that a transgenic organism is one in which genes
have been incorporated from a source other than its parents, ie there is a
transfer of genetic material from one species to another.[13]
2.8
Apart from viruses, all living
things are made up of cells or small structures bound by a membrane and filled
with a solution of interacting chemicals.[14]
Biological instructions are necessary for an organism to reproduce itself and
to produce the substances-proteins-required for it to function. These
instructions are encoded in a substance called deoxyribonucleic acid[15], or DNA for short.
2.9
DNA is a complex chemical
molecule called a polymer (‘having many parts’) a beaded string-like chemical structure
that is made up of many smaller chemical units. These smaller parts are called
nucleotides and are themselves comprised of three elements: a sugar, a
phosphate group and a ring structure of nitrogen and carbon, called a base.
There are four bases called adenine (A), guanine (G), thymine (T) and cytosine
(C). A DNA molecule comprises two strands of a number of nucleotides joined
together. The two strands are wrapped around each other to form a double helix.
The sugar and phosphate parts form the backbone of the DNA molecule, with the
bases facing inwards like the rungs of a ladder (see below). The chemical
characteristics of the bases are such that the adenine binds to thymine and
cytosine binds to guanine across the ladder.
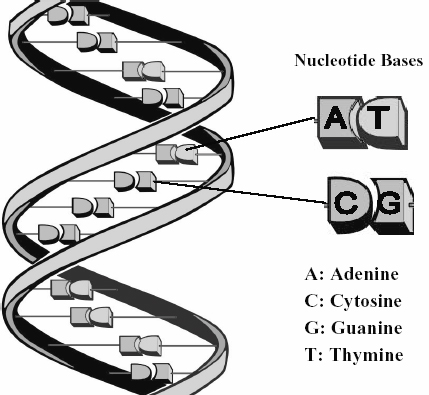
Figure 1: Diagram showing double helix
structure of a DNA molecule
2.10
The pairing of bases, known as
complementary base pairing, is an important feature of the double helix because
it means that if you know the order of bases on one strand, you can determine
the order on the other-something that is crucial to ensuring that the integrity
of genetic information is retained during the replication of DNA during cell
division and during the production of proteins. This raises concerns with the
Committee in terms of the addition of new genetic material during the genetic
modification process.
Genes and gene expression
2.11
A gene is a discrete segment of
DNA that provides the information necessary for synthesising a particular
protein at the right time and place, enabling an organism to function. The
genetic information is determined by the sequence of bases in the DNA.
2.12
An important component of a
gene is a sequence of DNA that occurs at the beginning of a gene, called the
promoter. The gene promoter determines whether the gene will be expressed in a
particular cell.
Gene
expression
2.13
Gene expression is the process
by which the biological information contained in genes is made available to
cells. During gene expression, one of the DNA strands is used as a template to
produce another molecule, RNA, or ribonucleic acid. This step is known as
transcription. During a second step known as translation, the RNA directs the
synthesis of proteins in accordance with the sequence of bases making up the
strand of RNA. The RNA contains sequence codes for 20 amino acids, which are
the building blocks of proteins.
Recombination
2.14
Recombination is the process
whereby new combinations of genetic material are formed by the techniques of
genetic engineering. There are three main applications of recombination used in
genetic engineering or modification:
-
the production of biologically useful proteins
to be used in the treatment of human medical conditions and in industrial
processes;
-
the modification of plants, primarily to provide
resistance to herbicides and insects attacks and resistance to infection by
viruses; and
-
the modification of animals to introduce new
traits.
2.15
The use of recombinant DNA
techniques allows variants of naturally occurring proteins to be produced.[16]
Selectable
markers
2.16
In order to verify that a
chosen gene has been incorporated into the DNA of the organism to be modified,
selectable marker genes are also often attached to the gene. These are
predominantly antibiotic resistance gene markers, but herbicide-resistance
genes also may be used as markers. The theory behind the use of these markers
is that, in the case of the antibiotic resistance markers, the gene confers
resistance to a specific antibiotic. If the organism into which the chosen gene
has been inserted is cultured in a medium containing that antibiotic, the
organism will survive if it has incorporated the new DNA which includes the
gene for antibiotic resistance. If the organism did not integrate the new DNA
into its own genome, it would not survive in the medium.
Plants
2.17
Cross breeding and grafting
have been used for centuries to produce hybrid plants by selectively crossing
plants with desired traits. Genetic engineering can now provide a direct method
for incorporating new traits into a plant.
2.18
One of the features of plants
that make them particularly suitable for genetic modification is that a whole
plant may be grown from a single genetically engineered cell. Two techniques
are used to transfer genes into plants. The first involves inserting a gene
from bacteria into a plant and the second, known as biolistics, is a procedure
whereby gold or tungsten balls are coated with DNA and fired into the plant
cell from a special gun. The DNA is released from the ball and integrates into
the plant DNA.
2.19
Goals of genetic modification
in plants include:
-
resistance to the attack of insects;
-
resistance to infection from viruses;
-
increased yield in food crops;
-
the ability to tolerate harsh environmental
conditions, for example, salinity.
2.20
To make a plant herbicide
tolerant, a bacterial form of an enzyme unaffected by a particular type of
herbicide, for example, gylphosate, is transferred into the plant. Two
approaches have been used to give plants insecticidal qualities. The first
involves transferring a gene from a bacteria that produces protein which is
toxic to some insects. The second technique genetically engineers the
expression of a protein to interfere with the insect’s ability to digest plant
tissue. Providing resistance to viruses has been achieved by introducing a gene
which encodes for a viral coat protein.
2.21
In addition to these qualities,
plants have also been engineered to delay ripening of fruits to increase shelf
life, alter colours in flowers, and improve the nutritional quality of crops.
Animals
2.22
While artificial selection, or
selective breeding, of animals has been used to produce domestic animals with
desirable traits such as increased milk yield, some desired traits cannot be
introduced without affecting existing ones. Transgenic animals can be produced
by the transfer of genes encoding the desired traits.
2.23
There are three techniques for
producing transgenic animals, all of which involve the genetic modification of
a fertilized egg sometimes called an early stage embryo. The modified embryos
are then transplanted into a host animal’s uterus. The first method involves
the use of a particular type of virus, called a retrovirus, which is used to
infect embryo cells. Microinjection is another method which involves injecting
DNA directly into the nucleus of the egg cell. Another method is through the
use of cells that are taken from the early stage of an embryo. These so-called
embryonic stem cells may be genetically modified before being reimplanted in
the animal.
2.24
Animals may be used in GMO
research, for example, the production of so-called ‘knockout mice’, that is,
mice which have been engineered to remove a gene to provide information on the
function of that gene. Another application is to use transgenic animals to
simulate human diseases which are the result of defective genes and to test new
drugs for their treatment, for example, in the case of arthritis and
Alzheimer’s disease. Finally, transgenic sheep and goats may be used to secret
recombinant human proteins in milk, including blood clotting factors and plasma
proteins.[17]
2.25
As well as the addition of
genes, genetic modification may involve the cancelling or augmenting of an
existing gene. Genes may also be activated artificially, for example by
spraying a crop with a specific chemical.[18]
2.26
Evidence presented to the
Committee raised a number of issues associated with gene technology and how it
should be regulated. While proponents of gene technology have claimed potential
benefits, opponents have also highlighted potential risks and the need to
ensure that adequate safeguards are in place to manage or eliminate these
risks.[19] These competing views are
discussed below, with references to other chapters where the regulatory
implications of these concerns are discussed.
Benefits associated with gene technology
2.27
Proponents of gene technology
cite its potential benefits for agriculture, the environment and human health.
Agriculture
2.28
The Interim Office of the Gene
Technology Regulator (IOGTR) argued that gene technology promises to be more
precise, produce results more quickly and cost effectively, and introduce
traits not possible through conventional techniques.
2.29
In relation to crop improvement, one of the major
benefits was seen to be the speed with which desired traits may be inserted
into the crop. AWB Ltd stated:
...the
process of wheat breeding has basically been going on ever since wheat was
introduced into Australia to develop certain quality characteristics such as
larger grains, better yielding grains in terms of flour extraction rates,
better frost tolerance, rust resistance and these sorts of things. That
breeding process has been continual. The time taken to do that through
traditional plant breeding methods is quite significant-eight to 10 years...What
gene technology will be doing will be taking those desirous genes from some of
those lines which are showing, for instance, rust resistance and putting those
genes into another type of wheat which shows a good quality flour product, for
instance, so that it has got both good quality flour and rust resistance, which
will be a much quicker process in terms of breeding than the traditional
approach of growing each of those plants out and selecting on a year-to-year
basis.[20]
2.30
Dr T J Higgins from CSIRO cited
an example of conventional breeding attempts to introduce rust resistance from
rye into wheat. While rust resistance was conferred on the plant offspring,
other undesirable genes were also transferred which led to the production of
sticky dough. Proponents of gene technology claim that gene technology is more
efficient than conventional techniques because only the desired gene is
transferred.[21]
2.31
While there may be risks
associated with transferring undesirable traits through conventional breeding,
a major concern about gene technology is not with the crossing of two of the
same plant species, but the transfer of genes from one species, for example a
fish, into another species such as a tomato, or a bacterium into a plant. This
ability to ‘cross the species boundary’ through genetic engineering introduces
an additional uncertainty and potential for serious harm. The ability of the
Gene Technology Bill to manage the risks posed by gene technology and ensure
that people and the environment are protected are discussed in Chapters 3 and 4
of this report.
2.32
The National Farmers’
Federation (NFF) identified a number of production benefits from crops derived
from gene technology including:
-
varieties with increased resistance to pests and
diseases which lead to benefits including reduced pesticide and herbicide use,
reduced input costs and reduced adverse environmental impacts from chemical
use;
-
new varieties which make better use of soil
nutrients, leading to reduced fertiliser use;
-
reduced labour costs and energy costs;
-
improved yields, quality and produce that is
better adapted to requirements of the food industry and consumers;
-
quicker adaptation of crops to environmental and
climatic factors, such as reduced water use, salt resistance and drought
tolerance;
-
crops which incorporate the nitrogen fixing
ability of lucerne, peas and soya into other crops, assisting improvement of
soil nutrition and enhancing productivity; and
-
accelerated breeding of plants with improved
characteristics leading to productivity gains, such as faster growing trees for
wood production and higher quality grains.[22]
2.33
Herbicide-resistance in crops
is a major objective of plant gene technology for reasons including:
-
increased production efficiency;
-
new options for weed management, such as
allowing flexible timing of herbicide application; and
-
decrease in overall herbicide use, leading to
increased use of more environmentally friendly herbicides, for example
glyphosphate.[23]
2.34
The NFF also referred to
potential benefits for consumers, including:
-
fruit and vegetables that keep fresh for longer,
reducing spoilage of food in transport and storage;
-
foods which contain healthy fats and oils and
cooking oils with lower saturated fat content;
-
increased nutritive value such as higher
expression of vitamins;
-
soybeans with a higher expression of anti-cancer
proteins naturally found in soybeans;
-
elimination of allergy-causing substances; and
-
food products which carry with them medicinal
properties.[24]
Environmental
2.35
The IOGTR outlined potential
benefits to the environment, including reducing the use of conventional
chemicals and pesticides. This would lead to more specific targeting of pests
and weeds, and reduce ground water contamination. Polluted or salt-affected
land could be reclaimed by the production of genetically modified salt-tolerant
crops, while higher agricultural productivity would reduce the need for land
clearing. Other potential benefits of gene technology are the cost-effective
production of biodegradable plastics and biodiesel, as well as the use of GMOs
for bio-remediation, for example, using micro-organisms to decompose toxic
substances and clean-up industrial sites or environmental accidents.
Health and
medical
2.36
As described earlier in the
chapter, gene technology also has been used in the areas of public health and
medical applications. A number of products are already being used in Australia,
including enzymes, hormones, blood coagulation factors, a Hepatitis B vaccine,
and a treatment for flu symptoms. IOGTR claimed that the advantages of these
products are improved efficacy, greater availability, cheaper production,
reduced allergenicity, and reduced risks of transmission of infectious agents.
2.37
Living GMOs have yet to be
introduced for therapeutic use in humans, however, it is claimed that they have
the potential to provide vaccines for cholera, malaria and HIV, and treatment
for cancer and diabetes.[25]
Risks associated with gene technology
2.38
While many potential benefits
of gene technology have been identified, evidence presented to the Committee
also highlighted a range of potential risks associated with genetically modified
organisms.
2.39
The IOGTR and others identified
risks arising from modern genetic manipulation techniques, especially
transferring genes from one species into a different species, including:
-
introduction of unidentified allergens into GM
food;
-
contamination of traditional or organic crops by
neighbouring GM crops;
-
the inability to eliminate a GMO once it is
released and found to have an adverse impact, as observed by the Organic
Federation of Australia (OFA):
Unlike chemicals in agriculture which are recallable and have a
half life and then eventually cease to be biologically active, GEO's are live
replicating organisms that once released, are likely to be [un]controllable;[26]
-
increased environmental damage due to increased
use of chemicals;
-
increased environmental competitiveness of GMOs
creating weeds, in the case of plants, or pests in the case of animals;
-
insect-resistant crops adversely affecting
non-target insects, exemplified by study of the impact of transgenic cotton on
the Monarch butterfly;[27] and
-
the transfer of genes for herbicide tolerance
from GM crops to related species resulting in herbicide-resistant weeds.[28]
2.40
In relation to the latter
point, Mr Scott Kinnear from the OFA advised:
...in
Canada...farmers have found cross-pollination, three canola crops resistant to
three types of chemicals...It will lead to increased use of that herbicide, and
it has to lead to increased use of that herbicide.[29]
2.41
Opponents have argued that
while the products of gene technology, such as herbicide resistant crops, long
shelf life melons and delayed ripening tomatoes, are likely to bring some
benefits to consumers, these products have been mainly developed to meet the
needs of those in the food supply system, growers, transporters, wholesalers
and retailers.
2.42
Notably, the crops that have
been subject to genetic engineering are those that are economically important
in the industrialised not the developing nations, for example maize, oilseed
rape (canola), sugarbeet, tomato and potato. Nevertheless some research and
trials have been conducted on wheat, rice, and cassava, an important food
source in African and South American countries.[30] Additionally, the main applications of
genetic modification are producing herbicide and pesticide resistant plants,
with much of the benefit going to the producers rather than consumers.
2.43
In referring to claims about
the potential environmental benefits of GM plants, Mr Phelps of the ACF
GeneEthics Network, stated:
There
are none with the existing crop on offer. Of all the releases to date,
70 per cent have been for herbicide tolerance by companies which also sell
the chemicals. They are selling farmer seed chemical packages, which intensify
the destruction being done to our environment. Our land and water are making us
so unsustainable that we are likely to have to be net importers of food and
fibre before long rather than exporters.[31]
2.44
The transfer of
herbicide-resistant genes from transgenic to wild or weedy relatives does occur
through cross pollination. The solution could require farmers to resort to
alternative, environmentally less friendly herbicides, and this would reduce
the attractiveness of growing the transgenic varieties. It has been argued that
‘controlled experiments cannot predict whether unexpected consequences will
occur’.[32]
2.45
The role of viruses in genetic modification, was
also raised in evidence to the Committee. Dr Dalling, from the companies
Florigene and Nugrain, indicated that viral ‘switches’ are used in the genetic
modification of carnations to produce violet varieties. He stated:
The
genes came from a range of other flowers in the first place-petunia or pansy.
Pansy was an important source of intense blue. There are genes in there though
that, from memory, have come from a construct or a part of a gene from a virus.
You might have picked up the term ‘35S’, which is a well-known regulator of
gene expression. To get genes to work you have to have a switch. One of the
more ubiquitous switches that is used commercially is 35S. It was isolated from
a virus back in the early 1980s. It has been the basis of a very large number
of constructs that have been used, not just by our company, but by other
companies around the world with currently released corn, soybean, cotton,
canola.[33]
2.46
However, virologist, Professor Adrian Gibbs,
expressed concern at the lack of research currently being conducted into the
consequences of using viruses for genetic modification purposes. He cited two
cases which he considered may cause serious problems:
I
put down two examples to mention to the committee: one is the development of
viruses for controlling mice by CSIRO division of wildlife research; and
another is putting virus genes into potatoes to try to control infection by
other viruses. Both of those technologies could result in major problems and,
as far as I know, there is no scientific work being done at present on the
safety to the environment of either of those developments. So I am worried
about the lack of research.[34]
Food
2.47
While there is greater
community acceptance of the use of gene technology in pharmaceuticals and
medicine, public concern related to GMOs in food remains high and increasing.
This has been expressed in calls for a ban or moratorium on all general
releases of GM crops and for clearer labelling of food products containing GMOs
or GM products.
2.48
The risks to human health of
greatest concern are:
-
transfer of allergens to new food products; and
-
the possibility of delayed effects similar to
CJD.
Antibiotic
resistance markers
2.49
The use of antibiotic
resistance markers in gene technology are controversial because of public fears
about the resistance trait transferring to bacteria in human and animal
stomachs. While studies have indicated that antibiotic resistance genes in
crops or crop products will have a negligible impact on food safety, there is
still a concern that the use of antibiotic resistance as a selectable marker
will ‘compromise the therapeutic use of antibiotics in humans and animals’.
Studies on the effect on food safety have shown, however, that ‘such transfer
occurs, if at all, at extremely low frequency’.[35]
2.50
Despite the conclusion of a
1996 report to the Nordic Council responsible for directing food policy issues
in five nordic countries, that ‘the overall risk is effectively zero, and that
the therapeutic use of antibiotics in humans or animals will not be affected by
commercialisation of transgenic crops containing antibiotic-resistance
selectable marker genes’, the London Royal Society in 1998 recommended that
antibiotic resistance markers should no longer be used in GM food crops.[36]
2.51
In evidence to the Committee,
Dr Tribe of the Australian Biotechnology Association, was critical of what he
considered to be an ‘overstated’ problem of antibiotic resistance markers.[37]
2.52
One of the reasons advanced for
using antibiotic resistance selectable markers is because of the inefficiency
of the techniques used to transfer DNA into host organisms, and the need to be
able to identify whether the target gene has actually been inserted into the
host cell. These markers can now be ‘zipped out’ leaving only the desired gene
in place.[38]
2.53
The Committee considers that
the potential risks associated with the transfer of antibiotic resistance genes
to other bacteria is another reason for ensuring extreme caution in the
regulation of GMOs, and this is discussed in detail in Chapter 4.
Allergens
2.54
The possibility that an
allergy-causing protein may inadvertently be transferred during the genetic
modification of a food product was raised in evidence to the Committee.[39] The dangers to human life that this
could pose led to the question of whether GM foods should be tested to the same
degree as medications. Dr Dalling from Florigene Ltd, responded:
In
principle I do not oppose it so long as all food is subject to the same
testing. At the moment anything that has the word ‘GM’ in front of it is
subject to the most unbelievable scrutiny. Long ago the concept of substantial
equivalence was well and truly established. I understand that people are
debating it now. A huge amount of evidence has been gathered to support the
idea, but it is an evolving process. More and more evidence may well be
demanded and gathered, presumably, so long as there is no discrimination as to
what the products are.[40]
2.55
Mr Buz Green of Serve-Ag, supported the stringent
testing of GMOs where there is a possibility of the transfer of allergens.[41] Mr Gary Burgess representing the South
Australian Farmers Federation, considered that issues of allergenicity in GM
products should be part of the risk assessment process.[42]
2.56
The Committee acknowledges that
there are concerns about the reliance on current scientific understanding to
identify risks, particularly given past experience when it was discovered that
scientific ‘fact’ turned out to be incorrect.
2.57
The case of the transfer of an allergen from the
Brazil nut into the soybean is a major concern. The case involved the transfer
of a protein gene from the Brazil nut into the soya bean to improve the quality of soya bean protein. After testing, it was discovered that the
gene caused allergic reactions in humans.[43] While the
Committee notes that in this case, the problem was identified before it had
been commercially released, the Committee considers that this is a serious risk
and that risk assessment processes must be rigorous enough to pick similar
instances up early. Risk assessment processes under the Gene Technology Bill
are discussed in Chapter 4 of this report.
Food
labelling
2.58
One of the areas that is
considered to be important in allowing consumers to make informed choices about
genetically modified food is the issue of food labelling. While a meeting of
New Zealand and Australian State and Territory Health Ministers in Wellington
in July this year discussed labelling of genetically modified foods, different
views were expressed in evidence to the Committee about the extent of labelling
required.[44]
2.59
The issue of food labelling is not covered by the
Gene Technology Bill, however, the Committee notes the important consumer links
between GM foods and labelling. One area of concern relates to the issue of substantial equivalence with respect to GM food products,
and how it effects how these products may be labelled.
Substantial
equivalence
2.60
Huppatz and Fitzgerald explain
the concept of substantial equivalence in foods as follows:
Substantial equivalence is established if food products are
essentially the same in composition, nutritive value, functional
characteristics and organoleptic properties (taste, smell, mouthfeel).[45]
2.61
If a genetically modified crop
is determined to be substantially equivalent to a conventionally grown crop,
‘the focus of testing becomes the introduced genes and their specific
products’, however, if the GM crop is not judged to be substantially
equivalent, then the crop must be ‘assessed for food safety on a case-by-case
basis’. Thus, for example, rice with enhanced vitamin A would be considered as
a ‘new food’.[46]
2.62
Dr Annison of the Australian Food and Grocery
Council (AFGC), explained how the concept of ‘substantial equivalence’ was
applied in food testing:
It
essentially says that, if we accept one product as being safe, the most
rational way of approaching assessing a second product it is to look for
differences from one to another. The principle of substantial equivalence looks
at the chemical composition and nutritive value and looks specifically for
levels of toxins and allergens. It compares one with another and determines
whether they are essentially the same. That seems to me to be a very practical
way to go...If there are different materials in foods, we also consider the
chances of their being bio-active in any way. We know that in some foods it
will be classified as substantially equivalent. There would be DNA in there
from the genetic modification. But there is no evidence whatsoever that DNA
itself, either from a genetic modification or just as we eat it, is biologically
active. In fact, we know it is not biologically active. We eat DNA all the
time, and we so know it is not biologically active. If there were an expression
production from that DNA present in any great quantity, it would be picked up
by the substantially equivalent definition anyway. That, on top of the tests
that are done by the companies who are developing these products, I believe
provides a very sound framework.[47]
2.63
A genetically modified product
that is deemed ‘substantially equivalent’ to its non-genetically modified
counterpart will not be labelled as a GMO.
2.64
In response to questions about whether the
products of cattle fed with GM crops should be considered GM, Mr Downer of the
AFGC replied ‘I would class them as GM free’. The AFGC added that:
...it
depends on exactly what you are feeding them, but if you are feeding them a
substantially equivalent GM crop-for example, if you are feeding them Roundup
ready soya beans as supposed to conventional soya beans, because they are
substantially equivalent; the differences between the soya beans are virtually
non-existent-there will be no differences in the animals feeding on those
crops. By definition, that is what ‘substantially equivalent’ means-there will
be no difference. So when you come to analyse the meat, you will not be able to
tell whether the meat came from an animal feeding on Roundup ready soya beans
or an animal feeding on conventional soya beans. This will be the difficulty
facing the retailers if they decide to go GM free and use that as one of the
stipulations: they could have two pieces of meat side by side and be making a
GM free claim about one, but there will be no way either the enforcement
agencies, in terms of making sure the label statements are correct, or, indeed,
the consumers buying the products, will be able to tell whether the label
statements are correct.[48]
2.65
Although there may be no
evidence of genetically modified DNA being transferred from GM crops through
the food chain, the public perception of this risk still exists.[49] The way in which consumer confidence
in gene technology can be enhanced is examined in Chapter 3.
2.66
The Committee notes that there is significant
disagreement about the nature and extent of the risks associated with genetic
engineering. The approach that should be taken with respect to the regulation
of GMOs in the light of the uncertainties and inconclusiveness about the
potential risks of gene technology are discussed in Chapter 3 of this
report under the section ‘the precautionary principle’.
GMOs covered by the Gene Technology Bill 2000
2.67
Another issue raised during the
inquiry was the way in which the Bill defines GMOs and gene technology. The
definitions of gene technology and genetically modified organism contained in
the Bill were referred to at the start of the chapter.
2.68
Heritage Seed Curators
expressed concern that regulations would be able to exclude organisms from the
definition of a GMO under the Bill.[50]
Friends of the Earth (Fitzroy) recommended that, in addition to the organisms
specified as GMOs in the Bill, the following should be added:
(d) any biological entity capable of replication or transfer of
genetic information, and includes plants, animals, bacteria and all other kinds
of micro-organisms, cell cultures (prokaryotic[51]
or eukaryotic[52]) created and propagated
as such, viruses, and plasmids[53] and
other kinds of vectors, in which the genetic material has been altered in away
that does not occur naturally, by means of cell or gene technology.[54]
2.69
One of the dangers in including
a list of additional biological entities under the definition of GMO is that in
providing such a prescriptive definition, the chance that something may slip
through may increase because the definition is too specific.
2.70
Concerns were raised about the
lack of regulation for stockfeed safety.[55]
However, the Committee notes that the draft regulations, released on 25 August,
declare that any GM product intended for use as a stockfeed is also a
genetically modified organism.
2.71
Under the Gene Technology Bill,
a GMO does not include:
-
a human being who has undergone somatic cell[56] gene therapy; or
-
an organism declared by the regulations not to
be a genetically modified organism, or that belongs to a class of organisms
declared by the regulations not to be genetically modified organisms.
2.72
The draft regulations exempt a
number of organisms listed from the Bill’s definition of a GMO because they:
-
give rise to organisms that can occur in nature;
-
are commonly used in biology; and
-
have a very long history of usage in Australia
and overseas.[57]
2.73
The IOGTR advised the Committee
that having chosen to define gene technology in broad terms in the legislation,
the exemptions in the regulations identify those techniques not generally
considered to be ‘gene technology’ that may have unintentionally been covered
by the Bill.[58]