Funding for research into low survival rate cancers
2.1
The impact of effective research investment is clearly demonstrated by
the increased survival rates for people with certain cancers, such as breast
and prostate cancer.[1]
Funding for cancer research comes from various sources, including the National
Health and Medical Research Council (NHMRC), which, as discussed further below,
recently restructured its grants program.[2]
2.2
This chapter commences by defining cancer research and then examines the
various sources of funding for such research, focussing specifically on
government funding through the NHMRC, Cancer Australia and the newly
established Medical Research Future Fund (MRFF), as well as philanthropic and pharmaceutical
funding.
2.3
The chapter then provides some context to the challenges facing funding
for research into low survival rate (LSR) cancers by providing an overview of
the Therapeutic Goods Administration (TGA), the Pharmaceutical Benefits
Advisory Committee (PBAC) and the Pharmaceutical Benefits Scheme (PBS).
The chapter concludes by examining the available funding for LSR cancers.
Cancer research
2.4
In 2016, Cancer Australia published a report into the funding for cancer
research projects in Australia from 2016–2018, using data from grants awarded to
these projects to the end of July 2015.[3]
2.5
This report identified that the Australian government is currently
funding 74 per cent, or $187 million, of the $252 million that has
been provided to 589 individual research projects for the period 2016–2018.[4]
Ninety five per cent of these research projects are funded by a single source.[5]
2.6
The following figure illustrates how cancer research funding for this
period has been allocated by reference to the Common Scientific Outline (CSO),
a system which 'uses easily applied terminology to describe and classify
research by where it best fits into the cancer research continuum'.[6]
Figure 1: The national pattern of cancer research funding
in 2016 to 2018[7]
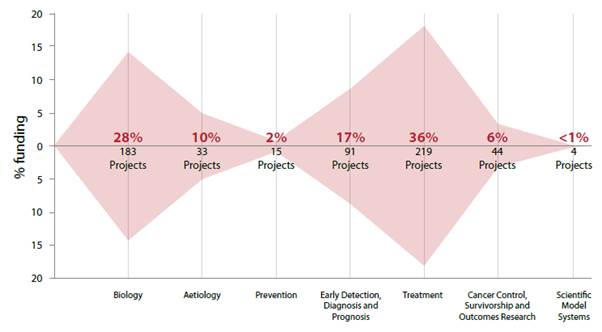
2.7
As can be seen in Figure 2, Cancer Australia also classified the cancer
research funding during this period by reference to a system developed by the United
States (US) National Cancer Institute, which is used to identify translational
elements within CSO sub‑categories.[8]
These categories are defined as follows:
-
Not Translational – basic
research;
-
Translational/Early – the
translational process that follows fundamental discovery and precedes
definitive, late-stage trials;
-
Translational/Clinical – research
at the clinical application end of the translational spectrum;
-
Translational/General – research
where difficulty in separating early and late translation/clinical research;
-
Translational/Patient-oriented –
research focussed on needs in the area of patient care and survivorship[9]
2.8
This figure illustrates that translational research in the clinical,
general and patient‑oriented categories will receive less than 10 per
cent funding each for the 2016–2018 period.
Figure 2: Percentage of funding to cancer research
projects and programs classified by translation categories[10]
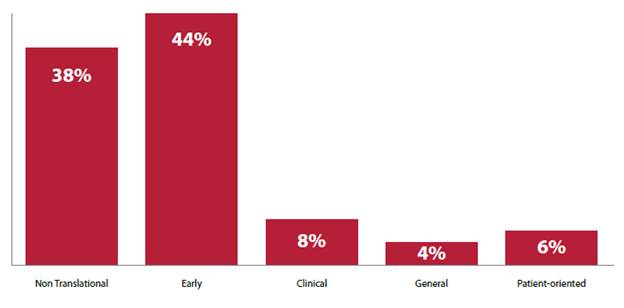
2.9
The lack of funding for the clinical stage of research was discussed by
the Low Cancer Survivals Alliance (LCSA), which submitted that '[t]here is a
lack of leadership by state and federal governments to encourage health
services to support clinical trial research':
Funding bodies such as the NHMRC traditionally do not support
translational research, therefore these breakthroughs are often not capitalised
on and further developed. Often funding for basic research is preferred over
clinical trials, as it can have more immediate results. As an example, in
February 2017 an incredible breakthrough was published in the international
journal Nature for the genome sequencing of pancreatic nets, led by Melbourne
University researchers. This research now needs to be supported and built upon,
in order for it to have an impact on patient outcomes.[11]
2.10
Indeed, The Unicorn Foundation similarly identified that 'the current
NHMRC model does not actively support translational research in low survival
cancers' and advocated for 'a new model of funding' for the NHMRC and support
for clinical trials for LSR cancers.[12]
2.11
However, in her evidence to the committee, Professor Anne Kelso of the
NHMRC made clear that her organisation funds discovery through to translational
research:
The NHMRC is interested in funding research that covers the
whole spectrum from discovery research, which might help us to understand the
origins of disease and also the origins of health, but we seek to fund across
the full spectrum from discovery through to translation into better health
care. We fund many clinical trials that assist in that translation of new
ideas, new discoveries, into better health care. We also fund research to
improve health services across the board. So there is a very broad range of
research that NHMRC funds, and some of it is very directly translational and
some of it is earlier stage.[13]
2.12
Despite this, Professor Stephen Fox identified a 'tension between true
translational clinical work and some of the basic discovery work', suggesting
how funding of clinical trials could jeopardise funding of discovery research:
There are the basic NHMRC studies, which are very much
discovery-type stuff, and then there is the other end of the spectrum, which is
the clinical trials-type activity. I think the clinical trials activity is
usually fairly explicit and straightforward in what the aims are. I think there
is an understanding behind that. The issue is that running a clinical trial, as
I am sure you have heard, is an incredibly expensive endeavour and takes a
large slice of the budget. So you only have to, I suppose, fund a few of those
and you have basically taken a huge chunk of your budget away from the
discovery sector.[14]
2.13
Advocating for a balance between discovery and translational research
funding, Professor Manuel Graeber identified that currently, 'there is no
balance' and further, that:
...translational outcomes, to some extent, represent marketing
speak. Politicians must be aware of the power they have. If the decision is
made to favour an area then everybody, in the current funding climate, will
jump at this. Administrators will and researchers have to follow but that is
wrong. Researchers are the ones that are supposed to come up with the
innovations. They are not being listened to often nowadays, because of the
way—based on a global trend—science has changed. In the old days it was just
idealists working somewhere without pay—some still work without pay today.
Generally institutions cannot afford it and that is the big problem—the
research dollars. I cost the university money. Teaching is much more
attractive, but, of course, it would be living on intellectual credit if we
would not support the research. That is the future.
I think it is really important how this is marketed—directed
by the politicians. Translational outcomes flies well with politicians, but it
is important to really look at the substance. What is really being produced?
Where is innovation coming from? How can we enable that? It will not come just
through some policy decisions. Scientists are not motivated to engage in it,
because it is like the 'fashion scientist', who makes a career by being in
policy making. We are about innovation. We are supposed to find new things that
are reproducible. That is our job. It is not to compete with politicians
implementing policies. That is my personal view, so do not blame it on the
university. That is my view, and I am happy to defend it.[15]
2.14
In its report, Cancer Australia concluded by identifying the following
opportunities for future strategic investment in cancer research, some of which
will be addressed in chapter 5 of this report:
-
targeted research investment by tumour site;
-
targeted research investment by research category;
-
translational research; and
-
research collaborations.[16]
Sources of funding
2.15
There are many different government and non-government sources of
funding for medical research. Although government funding can include funding
directly from the Department of Health (DoH), this chapter exclusively examines
funding from the NHMRC, Cancer Australia and the MRFF, which were the
government sources most frequently referred to in submissions and evidence to
the committee. At points throughout this report, there may be references to
other sources of government funding.
2.16
In addition to government funding for medical research, a significant
amount of funding is also provided by non‑government sources,
particularly philanthropic and pharmaceutical sources. For this reason, this
section also briefly examines these sources of funding.
The National Health and Medical
Research Council
2.17
The function of the NHMRC, a statutory body which operates pursuant to
the National Health and Medical Research Council Act 1992 (NHMRC Act), is
to assist the Chief Executive Officer (CEO) of the NHMRC, a position currently
held by Professor Kelso, in the performance of her functions.[17]
These functions are:
- in the name of the NHMRC, to inquire into, issue
guidelines on, and advise the community on, matters relating to:
- the improvement of health;
and
- the prevention, diagnosis
and treatment of disease; and
- the provision of health
care; and
- public health research and
medical research; and
- ethical issues relating to
health; and
- to advise, and make recommendations to, the
Commonwealth, the States and the Territories on the matters referred to in
paragraph (a); and
- to make recommendations to the Minister on expenditure:
- on public health research and
training; and
- on medical research and
training;
including recommendations on the
application of the Account; and
- any other functions conferred on the CEO in writing by
the Minister; and
- any other functions conferred on the CEO by this Act,
the regulations or any other law; and
- any functions incidental to any of the foregoing.[18]
2.18
The minister may also delegate additional functions to the CEO.[19]
2.19
The Council of the NHMRC[20]
provides advice to the CEO in relation to the performance of these functions,
and also performs any other functions conferred by the minister, the NHMRC Act,
its regulations, or any other law.[21]
2.20
Mr Greg Mullins of Research Australia observed that NHMRC funding has been
'effectively flatlining in recent years'[22]
and spoke to the positive effects of adequately funding the NHMRC:
One of the things that happened with NHMRC funding in the
period from 2000 to about 2010 was that the funding was doubled, and then it
was doubled again. That was a great outcome; it was really good news for the
sector. What it has done is attract a whole lot more people into the field. We
are seeing more people undertaking PhDs in this area. I think the latest budget
figures were predicting that Australia-wide we were going to move from 9½
thousand PhD completions last year to 12½ thousand by 2019-20. So we are seeing
an increasing number of people coming into this area.[23]
2.21
However, Mr Mullins opined that the availability of NHMRC funding to
support these researchers and their work is lacking, which is consequently
reflected 'in things like the drop in the success rates with NHMRC funding'.[24]
The difficulty of securing NHMRC funding was also identified by Dr Bryan Day,
who informed the committee that 'the competition in the current NHMRC funding
pool is incredibly high, because the pot of money is small'.[25]
The NHMRC's previous approach to
funding
2.22
The NHMRC is 'the largest single funder of health and medical research
in Australia', covering 'the breadth of health and medical research needs'.[26]
In its submission, the NHMRC set out the process by which it considers funding
applications:
Consistent with the NHMRC Act, NHMRC focuses on the relevance
of research proposals for health, rather than defining ‘health and medical
research’ as a set of research disciplines. NHMRC will fund research in any or
all areas relevant to health. It will also accept grant applications in any
research discipline and applicants are provided with an opportunity within
their application to explain how their research will lead to improved outcomes
in health.
Most NHMRC funding is awarded in response to
investigator-initiated applications in which the research is conceived and
developed by the researchers. A smaller proportion of funding is directed to
specific areas of unmet need, e.g., through Targeted Calls for Research,
special Centres of Research Excellence, Partnership Centres and some
Partnership Projects.
The primary criterion for all funding decisions is excellence.
NHMRC relies on review by independent experts to identify the best
applications, based on the significance of the research, the quality and
feasibility of the research proposal, and the track record of the
investigators. Rigorous processes of expert review ensure transparency, probity
and fairness.
When applications for funding are received, the office of
NHMRC manages the expert assessment of applications by independent experts. The
outcomes of expert review are used to determine which applications will be
recommended for funding. NHMRC’s [Research Committee] recommends those
applications to be funded through NHMRC Council to the CEO who submits them for
approval to the Minister with portfolio responsibility for NHMRC.[27]
2.23
The NHMRC also outlined its capacity to direct funding to priorities, as
required:
NHMRC’s range of funding schemes also provides the
flexibility necessary for targeting research and capacity building in key areas
of need in the health system. Each year NHMRC sets aside a component of the [Medical
Research Endowment Account] to address identified priorities. Priorities are
often implemented through additional funding provided for existing NHMRC
schemes, such as the Centres of Research Excellence scheme.
Each year, a small proportion of the total annual expenditure
budget is set aside to fund priority research areas through its Targeted Calls
for Research (TCR) funding program. A TCR is a specific funding mechanism that
invites grant applications to address a specific health issue. NHMRC may
initiate a TCR to address additional major issues that arise or in cases where
substantial gaps in evidence are identified. The aim of a TCR is to stimulate
or greatly advance research in a particular area of health and medical science
that will benefit the health of Australians. Through the TCR program, NHMRC has
an opportunity to identify and subsequently fund emerging health problems in
Australia.[28]
2.24
In respect of cancer funding in particular, the NHMRC stated that it 'is
the biggest funder of cancer research in Australia, accounting for 56% of all
funding nationwide'.[29]
The allocation of cancer research funding:
...is based on the review of each grant against a range of
investigator-provided data classifications including Burden of Disease
allocations, fields of research, keywords, grant titles and media summaries.
Many grants address more than one cancer type and in these cases the full value
of each is attributed to each relevant cancer type.[30]
2.25
The following table sets out the NHMRC's funding for cancer research for
the period 2012 to 2016, across all grant types, where the allocation of
funding is:
...based on the review of each individual grant against a range
of investigator provided data classifications including Burden of Disease
allocations, fields of research, keywords, grant titles and media summaries.
Many grants address more than one cancer type and in these cases the full value
of each is attributed to each relevant cancer type.[31]
Table 1: NHMRC cancer
research expenditure 2012 to 2016[32]
| Cancer Type |
2012 |
2013 |
2014 |
2015 |
2016 |
Total |
| Leukaemia |
$23,803,468 |
$19,769,414 |
$24,096,017 |
$25,068,518 |
$23,704,073 |
$116,441,490 |
| Breast
Cancer |
$24,803,186 |
$21,852,140 |
$20,508,426 |
$23,924,737 |
$21,469,127 |
$112,557,616 |
| Colorectal
Cancer |
$17,110,467 |
$14,400,726 |
$11,047,089 |
$13,427,898 |
$12,371,421 |
$68,357,601 |
| Childhood
Cancer |
$13,873,871 |
$12,425,114 |
$11,839,850 |
$12,219,439 |
$10,358,657 |
$60,716,931 |
| Melanoma |
$11,083,287 |
$11,012,931 |
$11,943,557 |
$13,145,930 |
$13,403,015 |
$60,588,720 |
| Prostate
Cancer |
$15,714,971 |
$10,777,957 |
$8,299,874 |
$8,895,471 |
$8,458,090 |
$52,146,363 |
| Hodgkin’s
Lymphoma |
$10,448,532 |
$8,507,097 |
$8,081,885 |
$8,088,540 |
$6,100,138 |
$41,226,192 |
| Ovarian
Cancer |
$11,516,436 |
$10,569,137 |
$7,690,016 |
$4,393,454 |
$4,701,048 |
$38,870,091 |
| Brain
Cancer |
$7,973,145 |
$7,207,891 |
$8,341,513 |
$8,469,035 |
$6,630,739 |
$38,622,323 |
| Lung
Cancer |
$5,822,566 |
$6,795,275 |
$7,610,659 |
$7,988,644 |
$7,769,633 |
$35,986,777 |
| Pancreatic
Cancer |
$9,812,427 |
$8,923,906 |
$6,841,808 |
$3,653,131 |
$4,117,523 |
$33,348,795 |
| Multiple
Myeloma |
$7,055,307 |
$6,079,353 |
$5,654,967 |
$5,851,116 |
$4,769,828 |
$29,410,571 |
| Liver
Cancer |
$3,209,094 |
$3,812,146 |
$5,470,925 |
$5,275,872 |
$4,455,742 |
$22,223,779 |
| Stomach
Cancer |
$3,731,366 |
$3,716,477 |
$2,662,717 |
$3,608,741 |
$4,695,318 |
$18,414,619 |
| Mesothelioma |
$1,914,182 |
$1,696,954 |
$2,097,639 |
$3,117,450 |
$2,142,460 |
$10,968,685 |
| Bone
Cancer |
$2,515,135 |
$1,986,772 |
$2,202,010 |
$2,205,394 |
$1,383,337 |
$10,292,648 |
| Oesophageal
Cancer |
$3,059,316 |
$2,667,775 |
$1,781,589 |
$1,524,016 |
$1,148,474 |
$10,181,170 |
| Endometrial
Cancer |
$2,362,829 |
$2,039,453 |
$1,587,515 |
$1,474,190 |
$1,420,730 |
$8,884,717 |
| Non-Hodgkin’s
Lymphoma |
$1,488,384 |
$1,533,322 |
$2,166,269 |
$2,210,672 |
$1,433,272 |
$8,831,919 |
| Head
and Neck Cancers |
$1,917,637 |
$1,929,367 |
$1,691,935 |
$1,195,252 |
$1,003,233 |
$7,737,424 |
| Cervical
Cancer |
$1,131,369 |
$1,442,060 |
$1,909,510 |
$1,040,493 |
$1,308,283 |
$6,831,715 |
| Testicular
Cancer |
$1,453,958 |
$1,602,101 |
$1,183,460 |
$1,194,662 |
$895,991 |
$6,330,172 |
| Kidney
Cancer |
$1,340,442 |
$852,278 |
$667,439 |
$420,627 |
$321,571 |
$3,602,357 |
| Bladder
Cancer |
$464,861 |
$467,727 |
$537,361 |
$304,437 |
$198,704 |
$1,973,090 |
| Thyroid
Cancer |
|
$97,733 |
$428,827 |
$551,373 |
$535,646 |
$1,613,579 |
| Vulvar
Cancer |
$439,249 |
|
$397,276 |
$383,721 |
$373,346 |
$1,593,592 |
| Adrenal
Cancer |
$295,384 |
$250,452 |
$119,529 |
$165,361 |
$477,340 |
$1,308,066 |
| Anal
Cancer |
$202,025 |
$132,337 |
$122,911 |
$60,173 |
|
$517,446 |
| Eye
Cancer |
$188,285 |
|
|
|
$36,134 |
$224,419 |
| Parathyroid
Cancer |
|
|
|
$124,531 |
|
$124,531 |
| Pituitary
Cancer |
|
$17,949 |
$38,437 |
$13,335 |
$21,197 |
$90,918 |
2.26
The NHMRC also provided the following additional table comparing its
research expenditure with incidence, mortality and survival rates, for 'all
persons', except in the case of the following gender-specific cancers:
cervical, ovarian, uterine, prostate and testicular cancers.[33]
The data for cancer incidence, mortality and survival rates were sourced from
the Australian Institute for Health and Welfare (AIHW).[34]
Table 2: NHMRC cancer research expenditure comparison
with incidence, mortality and survival rates[35]
| Cancer
Type |
NHMRC
Expenditure 2012 to 2016 |
2013
Age-standardised incidence rate |
2014
Age-standardised 5 yr mortality rate |
Five-year
relative survival from selected cancers, 2009–2013 (%) |
| Leukaemia |
$116,441,490 |
13.3 |
6.2 |
- |
| Breast
Cancer |
$112,557,616 |
63.6 |
10.5 |
90.2 |
| Colorectal
Cancer |
$68,357,601 |
57.7 |
14.9 |
68.7 |
| Melanoma |
$60,588,720 |
50.3 |
5.5 |
90.4 |
| Prostate
Cancer |
$52,146,363 |
151.3 |
25.8 |
94.5 |
| Hodgkins
Lymphoma |
$41,226,192 |
2.6 |
0.4 |
87.5 |
| Ovarian
Cancer |
$38,870,091 |
10.6 |
6.8 |
44.4 |
| Brain
Cancer |
$38,622,323 |
6.5 |
5.3 |
22.1 |
| Lung
Cancer |
$35,986,777 |
42.6 |
30.5 |
15.8 |
| Pancreatic
Cancer |
$33,348,795 |
10.9 |
9.3 |
7.7 |
| Multiple
Myeloma |
$29,410,571 |
6.3 |
3.3 |
48.5 |
| Liver
Cancer |
$22,223,779 |
6.9 |
6.4 |
17.3 |
| Stomach Cancer |
$18,414,619 |
8.1 |
4.2 |
28.5 |
| Uterine
Cancer |
$12,351,703 |
18.6 |
3.4 |
83.2 |
| Mesothelioma |
$10,968,685 |
2.7 |
2.6 |
5.8 |
| Bone
Cancer |
$10,292,648 |
0.8 |
0.4 |
69.7 |
| Oesophageal
Cancer |
$10,181,170 |
5.4 |
4.4 |
20.1 |
| Non-Hodgkins
Lymphoma |
$8,831,919 |
19.4 |
5.5 |
74.3 |
| Head
and Neck Cancers |
$7,737,424 |
17.2 |
3.8 |
- |
| Cervical
Cancer |
$6,831,715 |
6.8 |
1.7 |
72.1 |
| Testicular
Cancer |
$6,330,172 |
6.4 |
0.2 |
97.9 |
| Kidney
Cancer |
$3,602,357 |
11.9 |
3.4 |
74.9 |
| Bladder
Cancer |
$1,973,090 |
9.7 |
3.7 |
53.3 |
| Thyroid
Cancer |
$1,613,579 |
10.6 |
0.5 |
96.1 |
| Anal
Cancer |
$517,446 |
1.5 |
0.4 |
67.1 |
Criticisms of the previous approach
with respect to funding research into LSR cancers
2.27
A number of submitters and witnesses criticised the former NHMRC funding
model—in place up until the minister's announcement on 25 May 2017—and its 'one
size fits all' approach[36]
asserting that it disadvantages,[37]
or is biased against,[38]
researchers into LSR cancers.
2.28
For example, the Children's Cancer Research Unit (CCRU) of The
Children's Hospital at Westmead outlined some issues that arise with respect to
receiving NHMRC grants for research into LSR cancers:
We believe that characteristics of low survival rate cancers
can make it more difficult for associated research grant proposals to be
considered “well designed (or to have) a near flawless design”. The fact that a
particular cancer is characterised by poor survival rates can reflect a more
limited research base, leading to less scientific knowledge. This can mean a
greater need for more open-ended research grant applications seeking to (for
example) identify treatment targets, or biomarkers of response. However, these
more open-ended proposals can be viewed by grant review committees and
reviewers as “fishing expeditions” that may be less likely to be considered to
have “objectives that are well-defined, highly coherent and strongly developed
(and be either) well designed (or have) a near flawless design”. Similarly, low
survival rate cancers may have fewer experimental models (cell lines, mouse and
other animal models) available for study. It can also be challenging to access
statistically informative and representative sample cohorts, or patient cohorts
for clinical trials. Reduced resources for research could therefore also lead
to reduced “scientific quality” and “significance and innovation” scores for
NHMRC project grant applications, as well as negatively impacting the team’s
“track record”. One of the most problematic issues is how the determination of
“an issue of great importance to human health” is made, as this judgement can
clearly be made according to various criteria. The association between lower
cancer incidence and reduced patient survival can mean that research into some
cancers with poor outcomes could be viewed as less “important”.[39]
2.29
The LCSA similarly outlined how this funding program disadvantages
'researchers investigating low survival cancers, who generally have less pilot
data or proof of concepts than those researching more common cancers with
better outcomes'.[40]
It submitted that '[t]he NHMRC is not a reliable method for many researchers
wishing to secure research funding for low survival cancers to get worthwhile
projects off the ground'.[41]
2.30
Dr Marina Pajic informed the committee of the difficulties with
obtaining NHMRC funding based on her experiences:
In order to get something to the standard that NHMRC requires
to really be competitive, that study pretty much needs to be 80 per cent
complete. You need to convince these reviewers that this grant is foolproof,
that it will work, and that is not really what research should be all about. It
is all about figuring out that, actually, maybe something will not work. That
in itself may then be an interesting result that you take further and develop
new ideas around. I guess philanthropic money is really where those sorts of
studies are currently done, and there is just not a lot of that money around. I
am talking about pancreatic cancer researchers in general. I am fortunate
enough to have the support of the Garvan Research Foundation, so I have been
able to get my studies to that level to get NHMRC and Cancer Australia funding
on occasion.[42]
2.31
The Australasian Leukaemia and Lymphoma Group (ALLG) noted that, in its
experience, the NHMRC model in place prior to 25 May 2017 'favour[ed] those
cancers that attract more non-government funding'. The ALLG observed that those
cancers which attract non-government funding, have elements of:
-
public “popularity” and
prominence;
-
commerciality i.e. where industry
has a vested interest in a commercial pipeline; and
-
potential commercialisation of
intellectual property.[43]
2.32
However, in its submission, Research Australia suggested another reason
why this correlation between non-government and NHMRC funding exists: that is, '[t]he
NHMRC typically only funds the direct costs of research, leaving the
organisation undertaking the research to meet the indirect research costs from
other sources', such as philanthropic funding.[44]
An explanation of this reasoning was provided:
As a consequence of the continuing under funding of indirect
research costs, researchers need to find other sources of funding for the
balance of the indirect costs. In the case of universities and medical research
institutes, these sources include their own funds and philanthropic funding;
some of the latter are directed towards supporting research into specific
diseases. The availability of funding from philanthropic sources to meet the
indirect costs of research can influence the types of research that an
organisation will undertake and the applications that it will make to the NHMRC
for funding. To the extent that there is more funding available from non‑government
sources to support research into a particular disease, this can lead to more
applications to the NHMRC for funding in that area. This can favour research
into areas that have strong philanthropic support. Conversely, areas of
research that receive relatively less funding from non‑government sources
can be less successful in the open, competitive grant schemes administered by
the NHMRC and other government funding agencies.[45]
2.33
In its submission to this committee, the Victorian Comprehensive Cancer
Centre (VCCC) also discussed the significance of philanthropic funding:
Philanthropic sources of funding are divided between patient
support services and grants for research and these funds can make a significant
impact on preliminary research activity. Higher levels of philanthropic funding
for the various charitable cancer foundations has typically been related to (i)
higher survival rate cancers, where survivors are active in fundraising to
“give back” to the field, and (ii) high incidence cancers, where a large pool
of affected individuals and families can be leveraged for philanthropic
donations. Low incidence and low survival cancers do not have these resources
and moreover, there may be social stigma related to the cancers, e.g. lung and
brain cancers.[46]
2.34
Although the VCCC did not consider that there was any 'systemic bias' in
the NHMRC model, asserting that '[t]he process of scoring to assess NHMRC
applications is rigorous and robust',[47]
it was acknowledged that:
...the success rates of applications reflect the far greater
pool of resources available to researchers working in certain areas, e.g.
breast cancer, that supports them being successful researchers who will in turn
have greater success at NHRMC, i.e. it is the funding of preliminary work,
which requires scientists, expendables and infrastructure, that results in a
high-scoring funding application. It is also this funding that can enhance
track record and demonstrate that a research group can complete the project.
This tends to be in the cancer types that have already shown research success
and improved outcomes (which are more noteworthy than failures in poor outcome
diseases), further compounding the disparity between highly-funded and
low-funded research.[48]
2.35
Research Australia therefore proposed that the government should fully
fund indirect costs of research on the basis that this:
...would allow more philanthropic funding to be directed to
support novel early stage research and early career researchers, in turn
helping to improve their chances of securing Australian Government competitive
grant funding.[49]
Changes to the NHMRC funding structure
2.36
On 28 January 2016, the NHMRC CEO, Professor Kelso, announced 'an over‑arching
review of the structure of NHMRC's grant program',[50]
which was considered necessary for a number of reasons.
2.37
One reason was the decrease in funding for most of the NHMRC's funding
schemes from 2012 to 2015,[51]
which created 'a hypercompetitive environment, and [maybe] lead to research
proposals targeting low survival rate cancers being increasingly
disadvantaged'.[52]
This is illustrated by the following example of the Project Grants scheme at
Figure 3.
Figure 3: Rising
application numbers and falling funding rates in the Project Grants scheme,
1980 – 2015[53]
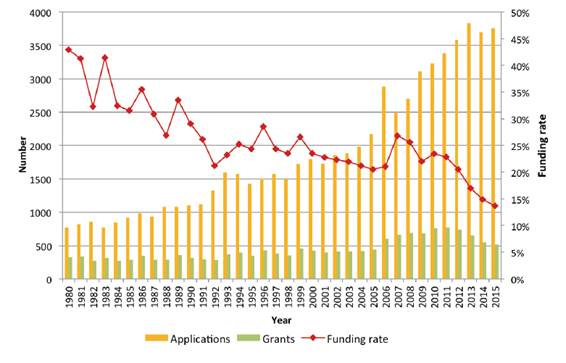
2.38
Further, there was also 'widespread concern that the high volume of
applications for NHMRC funding is having a range of negative effects on
Australian health and medical research' including that:
-
Researchers are spending a
substantial period each year preparing grant applications that will not be
funded, despite many being of sufficient quality to be funded.
-
The load on peer reviewers (most
of whom are themselves researchers) has become excessive for the number of
grants funded.
-
Early and mid-career researchers,
especially women, may feel discouraged from pursuing a research career.
-
Applicants are more likely to
propose, and peer reviewers are more likely to favour, “safe” research to the
detriment of innovation.
-
The low likelihood of funding is
driving further increases in application numbers as researchers seek to improve
their chances of obtaining a grant, exacerbating the situation.[54]
2.39
The NHMRC’s Research Committee, after considering a range of options,
reached the conclusion 'that commonly suggested changes to existing funding
schemes would not achieve a sufficient reduction in application numbers' that
would overcome such issues.[55]
2.40
Indeed, in 2015, many submitters to the NHMRC's public consultation on
Current and Emerging Issues for NHMRC Fellowship Schemes called for an overarching
review of the NHMRC's grant program.[56]
2.41
The review therefore had the aim of determining:
...whether the suite of funding schemes can be streamlined and
adapted to current circumstances, while continuing to support the best Australian
research and researchers for the benefit of human health.[57]
2.42
On 14 July 2016, the NHMRC released a public consultation paper on the review,
and public forums were also held in several capital cities.[58]
2.43
During the process of the NHMRC's review into its funding structure, an Expert Advisory
Group 'provided advice and assistance to NHMRC in examining the current grant
program and possible alternative models'.[59]
The CEO subsequently drew on its advice in formulating the new funding
structure, as well as that of the NHMRC Research Committee, the NHMRC Council,
Health Translation Advisory Committee, Health Innovation Advisory Committee and
the Principal Committee Indigenous Caucus.[60]
2.44
The NHMRC's restructured funding program, an overview of which appears
at Table 3, was announced on 25 May 2017[61]
and aims to:
-
encourage greater creativity and
innovation in research,
-
provide opportunities for talented
researchers at all career stages to contribute to the improvement of human
health, and
-
minimise the burden on researchers
of application and peer review so that researchers can spend more time
producing high quality research.[62]
2.45
In summary:
The restructured program will comprise Investigator Grants,
Synergy Grants, Ideas Grants and Strategic and Leveraging Grants. Limits will
also be placed on the number of grants an individual researcher can apply for
or hold.
Investigator Grants, Synergy Grants and Ideas Grants will
replace Fellowships, Program Grants and Project Grants[63]
Table 3: Overview of NHMRC's restructured grant program[64]
| Grant type |
Investigator Grants |
Synergy Grants |
Ideas Grants |
Strategic and Leveraging Grants |
| Purpose |
To support the research programs of
outstanding investigators at all career stages |
To support outstanding
multidisciplinary teams of investigators to work together to answer major
questions that cannot be answered by a single investigator. |
To support focussed innovative
research projects addressing a specific question |
To support research that addresses
identified national needs |
| Duration |
5 years |
5 years |
Up to 5 years |
Varies with scheme |
| Number of
Chief Investigators |
1 |
4-10 |
1-10 |
Dependent on
individual scheme |
| Funding |
Research support package (RSP) plus
optional salary support |
Grant of a set budget ($5 million) |
Based on the requested budget for
research support |
Dependent on individual scheme |
| Maximum number of applications allowed
per round* |
1 |
1 |
2 |
Not capped relative to Investigator,
Synergy and Ideas Grants. Dependent on individual scheme. |
| Maximum number of each grant type that
can be held** |
1 |
1 |
Up to 2** |
Not capped relative to Investigator,
Synergy and Ideas Grants. Dependent on individual scheme. |
| Indicative MREA allocation |
About 40% |
About 5% |
About 25% |
About 30% |
**
A maximum of two grants can be held concurrently, by any individual, with the
following exceptions and conditions: (1) individuals who hold two Ideas Grants
can hold concurrently a Synergy Grant, (2) individuals who hold up to two Ideas
Grants can apply for, and hold an Investigator Grant, but their RSP will be
discounted until the Ideas Grant/s have ended and (3) individuals may apply for
an Investigator Grant concurrently with an Ideas Grant, and if both
applications are successful only the Investigator Grant will be awarded.
2.46
In speaking specifically to the Ideas Grants, Professor Kelso informed
the committee that this scheme replaces some of what the Projects Grants scheme
achieved, 'but in a more effective way'.[65]
Professor Kelso continued:
The purpose of this scheme is to focus on research which is
highly innovative, creative and does not require that somebody has a long track
record of research, which is an impediment for many people getting started,
attempting to change fields or addressing an important new question. Of course,
it's still going to be highly competitive, it's going to be highly rigorous but
it will have a different flavour from the current Project Grants scheme, which
has become increasingly competitive, such that people's track records have
become a very important driver in that scheme. So I'm very optimistic that the
Ideas Grants scheme is going to fill an important gap in our current range of
schemes.[66]
2.47
Dr David Whiteman of the QIMR Berghofer Medical Research Institute
welcomed that the Ideas Grants were 'less focussed on track record and more
focussed on innovation', and acknowledged that while it is not a large pool of
money, 'it is a pool of money to address the issue of innovation and ensure
that innovative cutting‑edge ideas from younger early-career investigators
get picked up'.[67]
2.48
In speaking to the new five year grants for research, Professor Linda Richards
considered this a significant improvement compared to the previous three-year
funding structure, noting that this:
...is a huge step forward for everybody in terms of the amount
of time writing grants and the amount of time reviewing grants and also the
amount of time it takes to do high-quality research. You cannot do this in a
three-year funding cycle. It is just too short, especially for an organ system
like the brain, because the work is slow and time-consuming and it takes time
to do quality research. One thing though is that the NHMRC does have a fourth
category, which is for targeted research, and I would implore you that brain
research, in particular brain cancer, is one of those areas that we should be
targeting in this country.[68]
2.49
Dr Jens Bunt elaborated:
It is really hard to get long research programs, because most
of the project grants are for three years. Sometimes setting up something
ambitious or that is more risky takes more time. For instance, even though we
did not have funding for it, we invested three years to develop a mouse model.
It took us three years to get the exact model to mimic certain cancer
development. It is really hard to get funding for those kinds of things and
sometimes you have to think far ahead and invest a lot in developing techniques
and novel ideas that do not really directly fit in a project realm. There is
always an assumption of a small group of people working on something that is
finished within a certain set time. Whereas we, especially with rare cancers,
because we do not know that much yet, need to really develop these things with
multiple people from multiple different disciplines to work on it. It is really
hard to get sufficient scientific funding for that. I think this would also
help. But at the moment we have to think in packages of three years, which
makes it harder.[69]
2.50
Ms Emma Raymond also informed the committee that Wesley Medical Research
had to cease collecting samples, identifying the lack of longevity of funding
as a problem:
The problem is that people give you the money to set
something up and give you the infrastructure and the equipment, but there is no
longevity, so there is no funding to continue what we are doing. I have seen a
lot of biobanks go out of business when they have lost their funding from the
NHMRC. The problem is that we have a duty of care to these patients. We have
collected their samples to help other patients. If we lose our funding, then we
have to basically shut the doors, which is what happened at [the University of
Queensland] with their brain bank.[70]
2.51
Research Australia, which postulated that the changes to the NHMRC
funding structure 'are positive for the subject of this inquiry',[71]
also spoke to the importance of secure long term funding for research. Research
Australia stated that in order to see the greatest outcomes, research must be
funded for an extended period of time, as '[r]esearch, by its nature, is a long
term prospect', and provided the following example:
...to develop a new drug, from the initial stages through to
the end, takes anywhere between 10 and 15 years and can cost up around $3 billion.
So these are very intensive processes that need support over a long period.[72]
2.52
Although the overall changes to the grant program have been welcomed by
some, Dr Elizabeth Johnson of the VCCC warned that the NHMRC's 'capacity to
support multidisciplinary research may have been reduced' by these changes,
explaining that:
The focus is shifting away a little bit from the old
fashioned program grants, where you got a number of multidisciplinary teams, a number
of different people who had come from different institutions, who worked
together to support a particular research initiative. They typically tended to
be a bit bigger. We have yet to see how the restructure plays out, but the
NHMRC funding structure might not now be the ideal support for the type of
multidisciplinary approach that we need to really tackle [survival rates]
properly.[73]
Cancer Australia
2.53
Cancer Australia, a statutory body established in 2006 pursuant to the Cancer
Australia Act 2006, is 'the lead national cancer control agency' and 'aims
to reduce the impact of cancer, address disparities and improve outcomes for
people affected by cancer by leading and coordinating national, evidence-based
interventions across the continuum of care'.[74]
2.54
Cancer Australia has the following functions:
- to provide national leadership in cancer control;
- to guide scientific improvements to cancer prevention,
treatment and care;
- to coordinate and liaise between the wide range of groups
and health care providers with an interest in cancer;
- to make recommendations to the Commonwealth Government
about cancer policy and priorities;
- to oversee a dedicated budget for research into cancer;
- to assist with the implementation of Commonwealth
Government policies and programs in cancer control;
- to provide financial assistance, out of money
appropriated by the Parliament, for research mentioned in paragraph (e) and for
the implementation of policies and programs mentioned in paragraph (f);
- any functions that the Minister, by writing, directs
Cancer Australia to perform.[75]
2.55
In its submission, Cancer Australia noted that it performs its function
to oversee a dedicated budget for research into cancer[76]
through administration of the Priority-driven Collaborative Cancer Research
Scheme (PdCCRS).
2.56
The PdCCRS, established in 2007, 'brings together government and other
funders of cancer research to coordinate, co-fund and maximise the number of
cancer research grants funded in Australia',[77]
and was established:
...in order to:
-
better coordinate funding of
priority-driven cancer research;
-
foster collaborative cancer
research and build Australia’s cancer research capacity, and
-
foster consumer participation in
cancer research, from design to implementation.[78]
2.57
In determining which research programs to fund, Cancer Australia uses
'an evidence based approach' to fill gaps in funding, which was described to
the committee by Dr Paul Jackson:
We look at the national pattern of funding to cancer
research, which includes the funding that is provided from both national and
international sources, and, using that profile, we examine the funding that
goes to different tumour types as well as the funding across the broad areas of
the research spectrum—the main areas of the funding to where that project goes.
We then use that evidence to identify opportunities for us to make strategic
investments where there are gaps or opportunities to further research. That,
for example, can be in tumours which may be of high burden and poor survival,
where there are opportunities to strategically invest to address that.[79]
2.58
Dr Jackson informed the committee that in determining which applications
to fund, a merits-based approach is used, such that Cancer Australia funds:
...from the top-ranked merit based application downwards. We
maximise the amount of funding, or the number of grants that we're able to
fund, through collaborative funding with our funding partners in the scheme. We
start from the top down. Once the funding has ended, that's where we have to
stop funding.[80]
2.59
Dr Whiteman commended Cancer Australia on this approach:
I think the activities that Cancer Australia has done in just
looking back and saying: 'What have we funded previously? Does that reflect
where we want to invest our funding?' are very helpful, because they then put
the spotlight on neglected areas of research, including low-survival cancers. I
think there is a mood for recognising where there are deficits in funding, and
then looking for mechanisms to correct that.[81]
2.60
Other witnesses described the type of funding they receive from
Cancer Australia, and the positive impact this has had on their research.[82]
For example, Ms Delaine Smith of the ALLG informed the committee that:
...the ALLG, and now 13 other cancer trial groups around
Australia, have been able to have funding come straight from Cancer Australia.
That is about half a million dollars a year. The infrastructure that it
supports is very specific because Cancer Australia is very specific about how
it can be spent. So it goes towards the activities that develop clinical
trials. For us, in the ALLG, we utilise that funding on EFT and on roles and
positions that help prepare the clinical trial protocol. The protocol is the
instruction document that is going to go to the hospital to tell them what to
do in a very methodical and meticulous way. You cannot understate the
importance of preparation. Preparation is key.[83]
2.61
However, the committee also heard that Cancer Australia could have a
lead role with respect to 'developing, implementing and maintaining' a
sustained focus on LSR cancers.[84]
Further discussion about a national strategy for LSR cancers appears at chapter
5.
The Medical Research Future Fund
2.62
The MRFF, which operates pursuant to the Medical Research Future Fund
Act 2015 (MRFF Act), was established as part of the 2014–15 Federal Budget
with the purpose of providing:
...a sustainable source of funding for vital medical research
over the medium to longer term. Through the MRFF, the Government will deliver a
major additional injection of funds into the health and medical research sector.[85]
2.63
The $20 billion fund 'offers the opportunity to strategically fund
research and address national priorities in a cohesive and coordinated way'.[86]
The MRFF 'complements existing medical research and innovation funding', such
as the NHMRC, the Commonwealth Science Council and the National Innovation and
Science Agenda, 'to improve health outcomes by distributing new funding in more
diverse ways to support stronger partnerships between researchers, healthcare
professionals, governments and the community'.[87]
2.64
The operation of the MRFF is summarised in the MRFF Act as follows:
The Medical Research Future Fund consists of the Medical
Research Future Fund Special Account and the investments of the Medical
Research Future Fund. Initially, the Fund’s investments are a portion of the
investments of the Health and Hospitals Fund which was established under theNation—building Funds
Act 2008. Additional amounts may also be credited to the Medical Research
Future Fund Special Account.
The Medical Research Future Fund Special Account can be
debited for 3 main purposes:
- channelling grants to the COAG Reform Fund to make grants
of financial assistance to States and Territories; and
- channelling grants to the MRFF Health Special Account to
make grants of financial assistance to certain bodies; and
- making grants of financial assistance directly to
corporate Commonwealth entities.
The Australian Medical Research Advisory Board is established
to determine the Australian Medical Research and Innovation Strategy and the
Australian Medical Research and Innovation Priorities. The Health Minister takes
the Priorities into account in making decisions about the financial assistance
that is provided from the Medical Research Future Fund Special Account.
There is a limit on the amount that can be debited from the
Medical Research Future Fund Special Account each financial year. The limit,
which is called the maximum annual distribution, is determined by the Future
Fund Board for each financial year.
The Medical Research Future Fund is invested by the Future
Fund Board in accordance with an Investment Mandate given by the responsible
Ministers.[88]
2.65
Professor Ian Frazer, Chair of the Australian Medical Research Advisory
Board (AMRAB) which determines the Australian Medical Research and Innovation
Strategy and the Australian Medical Research and Innovation Priorities pursuant
to the MRFF Act,[89]
outlined for the committee the differences between the NHMRC and the MRFF:
The National Health and Medical Research Council largely
gives funding out in reply to specific proposals from individual researchers.
It does have some priority areas which it uses, but the vast majority of
funding is in response to a particular proposal on a particular bit of research
determined by the investigator themselves. The Australian Medical Research
Advisory Board advisory to the Medical Research Future Fund rather takes the
view of top-down driven research where we have recommended to the minister
priorities where we believe that research money should be best spent.
Therefore, while there might be a call for proposals in due course, at the
moment the money is being dispersed on the basis of the priorities and
strategies that we set when we completed our consultation with the medical
research community, the general public and other interested parties in the
course of 2016.[90]
2.66
Professor Frazer considered that the MRFF Act provides sufficient
flexibility in the granting of funding, specifically in relation to
collaboration across institutions:
Certainly, the funding will have to be administered by one
individual organisation which is responsible for its acquittal back to
government. But the concept of collaboration in research is pretty much
international, of course. Certainly, there is nothing intended about the way
that we made the strategy of priorities to suggest that we did not wish to see collaboration.
In fact, we positively expected that there would be collaboration and pointed
out that the value of collaboration, for example, between different research
institutes in this country and overseas, and research institutes and industry,
should be positively encouraged.[91]
The 2016–2021 strategy
2.67
Following consultation with the sector and the broader community, and
pursuant to the MRFF Act,[92]
the AMRAB developed six strategic platforms to underpin the Australian
Medical Research and Innovation Strategy 2016–2021 (the Strategy) that 'capture
and group together themes and provide a framework for the [Australian
Medical Research and Innovation Priorities 2016–2018] to improve research
capacity and capabilities in the research sector'.[93]
A list of priorities falls under each of these strategic platforms.[94]
2.68
The Strategy also sets out how the MRFF aligns with and compliments the
NHMRC, the National Science and Innovation Agenda, and other interests, such as
state and territory governments and the private and not-for-profit sectors;[95]
as well as the challenges facing the health and medical research sector.[96]
2.69
The strategic platforms of the Strategy are:
-
strategic and international horizons: funding to support
Australian participation and leadership in 'international research projects
focusing on major global health challenges and threats...complimentary to the
international collaborative activities of the NHMRC';[97]
-
data and infrastructure: funding for research that 'enables
the planning and implementation' of 'an integrated national health data
framework that supports healthcare delivery, service improvement and best
practice adoption';[98]
-
health services and systems: in contrast to the current
product and drug focussed medical research and the domination of the acute care
experience for research on health interventions, the intention is to bolster 'Australia’s
capacity in health services and systems research' by, for example, 'investment
activities...with the Medicare Benefits Schedule Review Taskforce and new policy
and program agendas, such as the Australian Government’s Health Care Homes
trial';[99]
-
capacity and collaboration: the focus is research
collaboration, to be achieved by 'investing in multi-disciplinary, institute
and sector teams', which could extend to collaborative funding, 'by leveraging
co-investment from other governments, private and philanthropic interests';[100]
-
trials and translation: the facilitation of 'non-commercial
clinical trials of potential significance', including by supporting
NHMRC-accredited Advanced Health Research and Translation Centres;[101]
and
-
commercialisation: supporting 'the creation and brokering
of linkages between researchers and industry that are transdisciplinary in
nature', noting the need for '[a] two-way exchange of knowledge and expertise
in research, and its translation into clinical practice' and better
encouragement 'adoption of the requirements for successful commercialisation in
both the academic and business environment'.[102]
2.70
Professor Frazer commented that, for the next round of consultations,
improvements could be made to AMRAB's processes:
...we may actually have to get focus groups together and
specifically engage, through the recruitment of individuals who would not
otherwise necessarily come forward, to get a more general representation of
what the public is interested in. One of the practical realities, of course, is
that people become most interested in the health system when they actually need
to use it, and yet the vast majority of people out there who might, in the
future, benefit from it, do not actually use it at the moment.[103]
2.71
Indeed, Professor Rosalie Viney of the Australian Health Economics
Society advocated for an additional injection of funds from the MRFF into
health research 'across the board':
It shouldn't just be in the discovery science; it needs to be
across the whole of translation. But I think it's absolutely critical that that
is done in a way that maintains the standards of excellence in research,
maintains the standards of scientific quality, makes sure that we apply the
same well-established principles that organisations like NHMRC have had for
peer review and for quality, and that that continues.[104]
2.72
However, Dr Richard De Abreu Lourenco warned that if the MRFF were to be
used for discovery research, it could be viewed 'as an implication of support
for commercialisation' from the government.[105]
First disbursements
2.73
The first disbursements of the MRFF, implemented in 2016–17, invested
$65.9 million:
-
$20 million for preventive health and research translation
projects.
-
$33 million for clinical trials that will build on Australia’s
world class research strengths and ensure Australia is a preferred destination
for research.
-
$12.9 million for breakthrough research investments that drive
cutting edge science and accelerate research into better and new treatments and
cures.[106]
2.74
Professor Terrance Johns of the Brain Cancer Discovery Collaborative,
who stated that his institution 'is not a large institution with political
clout', noted that '[t]here was no call for grants for MRFF funding' for its
first disbursements, and observed that the funds are 'pretty much locked up by
the G8 universities'.[107]
Professor Johns opined that, at present, the MRFF 'is about political
clout'.[108]
2.75
However, Mr Peter Orchard, whose organisation CanTeen Australia was a
recipient of some MRFF funding, suggested that '[t]o some extent, the MRFF is
in its absolute infancy, and so being able to comment on it feels difficult at
this stage, other than to say I am very grateful for it'.[109]
2.76
Indeed, Mr Mullins of Research Australia spoke to the benefits of the
MRFF:
...the MRFF funding, with its emphasis on translation, offers
new opportunity for advances that will benefit patients. The MRFF, importantly,
also has a top-down approach to funding. It is driven by a five-year strategy
and priorities, and the latter must explicitly take into account the burden of
disease, how to deliver practical benefits to the Australian community and
value for money. This must be combined with a focus on funding excellent
research, obviously, if it is to be successful, but it provides greater scope
for strategically directing funding to particular areas.[110]
Philanthropic funding
2.77
As indicated at paragraphs 2.32–2.33 above, philanthropic funding can be
vital to advances for research into LSR cancers, especially when researchers
find it difficult to obtain government funding.
2.78
Indeed, it was noted by the ANZCHOG National Patient and Carer Advisory
Group that 'oncology units are often largely dependent upon philanthropic and charitable
donations' to meet costs associated with enrolment in and compliance with
international trials, emphasising that '[c]urrently paediatric centres rely
heavily on philanthropy, charities and individual hospital budgets to fund most
cancer clinical trials'.[111]
2.79
To illustrate what such funding can achieve, the Mark Hughes Foundation
(MHF) outlined that in three years, it has contributed to the following
improvements in respect of brain cancer:
-
A Brain Cancer Biobank at [the Hunter
Medical Research Institute]
-
Over $300,000 in project grant
funding and various Travel Grants to allow brain cancer researchers attend
international conferences to present their work and establish important
research collaborations
-
A clinical research fellowship in
Brain Cancer
-
A dedicated Brain Cancer Care
Nurse at John Hunter Hospital
-
Communal brain cancer research
register with Brain Cancer Biobanking Australia[112]
2.80
Further, Professor Mark Rosenthal of the VCCC spoke to the work of the Cure
Brain Cancer Foundation (CBCF), a philanthropic organisation focused
exclusively on brain cancer, in providing financial assistance for brain cancer
research:
The [CBCF] has done terrifically well through, really, one
individual driving that over many years, but they now have a very established
philanthropic organisation that runs professionally and relatively
independently. We have made sure that there is rigour to their grant
application process and the grants that have been given out. It is not in
competition with NHMRC. It has grown because of the need for it. It would be
great if we did not have to have philanthropic funding, but actually we are
lucky in brain that at least there is some. We have only had one round of
grants, which total up to $2 million, I think.[113]
2.81
However, Associate Professor Gavin Wright identified a significant issue
with attracting philanthropic funding for LSR cancers, namely, the lack of
survivors:
The trouble with the philanthropic side of things is often
you need survivors, who generate a lot of push for these sorts of things. They
go to companies. The catch 22 is that, if you have a poor-survival cancer, you
do not have many survivors. If it is affecting a lower socioeconomic group, you
do not have the movers and shakers.[114]
2.82
Furthermore, as Dr Johnson noted, 'success breeds success' in terms of
the growth of philanthropic cancer support groups, observing that:
Once you have a critical mass of funding you can then do more
with it—you can advertise more and you can grow your foundations more. There
are numerous lesser-known small cancer foundations which really do exist on the
smell of an oily rag.[115]
2.83
The committee therefore heard calls for various improvements in respect
of philanthropic funding. For example, in addition to the recommendation by
Research Australia at paragraph 2.35 above that the government fund indirect
costs of research in order to 'allow more philanthropic funding to be directed
to support novel early stage research and early career researchers',[116]
Professor Guy Eslick called for greater philanthropy from 'wealthy
Australian businesses and individuals'.[117]
2.84
In his submission, Professor Eslick drew a contrast between the philanthropic
funding Harvard University received for research during his post-doctoral
training at Harvard ($100 million), compared to that received by the University
of Sydney in that same week ($10 million).[118]
Professor Eslick suggested that the government could encourage philanthropists
to donate to universities and research institutions by offering greater
incentives.[119]
2.85
The committee also received the following suggestions for improvement
with respect of philanthropic funding:
-
the Lung Foundation Australia called for the '[p]hilanthropic
community to establish specific targets for donations to lung cancer research';[120]
-
the MHF called for '[t]argeted Federal and state funding towards
brain tumour research, leveraged with funds from philanthropic agencies' to enhance
productivity in the field of brain cancer research;[121]
and
-
Ovarian Cancer Australia recommended the development of 'a
national strategy for coordinating the planning and funding of cancer research
across the government, medical, health, research and philanthropic
communities'.[122]
2.86
Despite the evidence from a number of submitters about their difficulty
in securing philanthropic funding, Mr Todd Harper of the Cancer Council
Victoria informed the committee that his organisation had not found it
difficult to get philanthropic support for research into LSR cancers, asserting
that:
...we have found that there is both an appetite amongst
philanthropy to invest in the haematology of less common cancers and in the
high-risk, high-return research. I think what is critical here though is that
one of the things that makes it more likely that philanthropy would fund these
is if they can have assurances over the quality or the rigour of the scientific
processes that assess those proposals. I think there is opportunity to bring
together the best scientific minds to assess high-quality proposals that can be
funded by philanthropic organisations like ours, or indeed others. I think
government can also play a role in providing seeding or cooperative funding to
enhance the chances of those programs being successful and the chances of those
programs being successfully funded.[123]
2.87
However, the committee also heard that '[p]hilanthropy will only go so
far': in speaking of the establishment of a centre for research excellence, although
the Walter and Eliza Hall Institute of Medical Research had benefitted from
philanthropic funding when NHMRC funding was not available, Professor Clare Scott
noted that '[g]overnment funding would allow us to entrench these approaches in
Australian medicine'. [124]
Pharmaceutical funding
2.88
A number of witnesses, whose clinical trial research was funded by
pharmaceutical companies, outlined for the committee the importance of funding
from pharmaceutical companies for cancer research.[125]
However, as the below evidence demonstrates, many witnesses were also critical
of the reluctance of pharmaceutical companies to become involved in drug
development for people with LSR cancers.
2.89
Roche Products Pty Limited (Roche), a research‑based healthcare
company focussing on pharmaceuticals and diagnostics, discussed the role of
pharmaceutical companies in improving survival rates for LSR cancers:
The pharmaceutical industry is a critical component of the
innovation ecosystem. Not only does industry contribute to basic research and
takes the lead in taking medicines through regulatory and reimbursement
processes, it is also the leading funder of clinical trials.[126]
2.90
Roche identified that improving survival outcomes for people with LSR
cancers is dependent on a number of factors including overcoming barriers to
participation in clinical trials (by clinicians as well as patients), and
affordable access to treatments through the PBS.[127]
Roche identified that '[b]reakthroughs in personalised medicine and
immunotherapy are offering hope to patients with both common and rare cancers –
yet these products face many challenges in navigating the reimbursement
system'.[128]
2.91
Indeed, a recent Deloitte Access Economics (Deloitte) report noted that
currently, 'only a small proportion of the potential indications for which
immunotherapies are able to be used in cancer treatment receive subsidised
funding from the Government', and as these therapies are expensive to develop
and produce, treatments 'are prohibitively expensive for many patients who seek
to self-fund'.[129]
A further discussion of this report, and its recommendations, appears at
chapter 5.
2.92
Medicines Australia—'the Australian peak body for the discovery-driven
pharmaceutical industry'—identified other challenges for pharmaceutical
companies particularly in respect of the policy and access environment:
The broader policy environment is also challenging the
investment decisions made by pharmaceutical companies. Increasing levels of uncertainty
caused by a single payer system, as well as inconsistent approaches to
intellectual property, aggressive pricing policies and an unpredictable policy
environment, are among the issues which Medicines Australia finds to be of some
concern.[130]
2.93
The committee also received evidence that there is a limited incentive
for pharmaceutical companies to fund clinical trials for LSR cancers,[131]
with one witness describing the lack of funding for brain tumour research 'very
disappointing'.[132]
Other barriers to clinical trials distinct from pharmaceutical funding that are
faced by people with LSR cancers is examined in chapter 3.
2.94
Speaking to the involvement of pharmaceutical companies in drug
development, Professor Richards asserted that 'it is unethical not to think about
those patients [with LSR cancers] and not to be trying to develop treatments
for them', arguing that '[t]hat is where government has to step in'.[133]
Professor Richards stated that:
...pharmaceutical companies have been turning away from drug
development for brain, partly because we, firstly, did not know enough about
the pathways involved to make the clinical trials effective. Also, for rare
diseases, of course, the market is not there for the company to want to invest
in a drug that is going to be used by a small number of patients.[134]
2.95
The ANZCHOG National Patient and Carer Advisory Group also recognised
the importance of return on investment for pharmaceutical companies, submitting
that '[t]here is little economic incentive for pharmaceutical companies to fund
paediatric cancer trials' as childhood cancers are 'made up of rare and
ultra-rare diseases'.[135]
2.96
This was also reflected by Mrs Therese Townsend, a pathology scientist who
has a neuro-endocrine tumour:
The costs of running such trials are disproportionate to the
potential profit when there are few potential “customers”. When those who may
benefit have inherently poor prognoses, courses of treatment are likely to be
short, and this further minimises the return on research investment. Hence there
is no financial incentive for private enterprise to conduct such trials,
especially in Australia due to its decentralisation and small population base.[136]
2.97
Dr Chris Fraser spoke to two barriers to participating in international
clinical trials: first is the cost of participation, and second, the increasing
requirement to partner with pharmaceutical companies.[137]
Dr Fraser elaborated on this second barrier:
Historically, this was very much an academic pursuit and
there were not new drugs, as I outlined, so we were able to do this amongst
ourselves. As these new drugs are developed, we increasingly have to partner
with pharma companies. Australia is not a big market. It is expensive for them
to open these trials in Australia. There may be only one, two or three
Australian patients that are eligible for a particular trial. So we need to
work out a structure that means we can still participate in these trials. The
first step to that is to make sure that we have a very robust clinical trials
infrastructure so that we are up and ready to start these trials so the
pharmaceutical companies know that the infrastructure and the organisations are
there to make sure that the process will run smoothly.[138]
2.98
Indeed, the Garvan Institute of Medical Research/The Kinghorn Cancer
Centre/The Garvan Research Foundation (Garvan Institute) identified that '[t]he
cost of drug development, which must be recouped by the pharmaceutical
industry, already limits access of some patients to important treatment
options' and outlined the significant cost of running trials:
The financial costs of conducting clinical trials have
doubled every nine years for the past 50 years. The estimated combined costs
per patient in a cancer clinical trial rose from less than US$10,000 to around
US$47,000 between 1980 and 2011. The average phase 2 study of 40 patients costs
upwards of US$2-10M, while the average phase 3 study costs upwards of US$40M.
Average development costs are estimated at around US$3.6 billion dollars per
drug.[139]
2.99
However, the Garvan Institute also informed the committee about the
alternative ways it has engaged with pharmaceutical companies to conduct
clinical trials. In order to minimise the barriers to engagement with
pharmaceutical partners in respect of its Molecular Screening and Therapeutics
(MoST) study, the Garvan Institute sought only:
...access to study drugs for each module and for engagement
with the pharmaceutical partner in data interpretation, as well as
decision-making regarding expansion of a drug–disease cohort in which a significant
signal of activity has been identified.[140]
2.100
Professor David Thomas of the Garvan Institute explained how this system
works in practice:
...we invest in drugs by where they are arise. If you invest in
breast cancer, you authorise and reimburse drugs on the basis that it works in
breast cancer, and that drives the way in which pharma invest. The problem is
that many of these drugs work across a whole range of cancers, because a whole
range of cancers have this particular common molecular abnormality. A molecular
taxonomy is required. That requires molecular screening. Pharma cannot invest
in screening 10,000 people to find 20 to treat, but we can. If we can match our
research investment with the opportunities from pharma, so we can create a
healthy model of collaboration with the benefit of pharma in mind but also
getting patients onto trials, that is a virtuous cycle.[141]
2.101
Further discussion about clinical trials appears at chapter 3, and
further discussion about the treatment of cancer through personalised medicine
and immunotherapies is found in chapter 5.
The TGA, PBAC and PBS
2.102
In order to understand the challenges that face people with LSR cancers,
and why those 30 per cent of cancer deaths in Australia that are 'a consequence
of the lack of investment in research' receive six per cent of all drug
funding,[142]
it is necessary to briefly examine the key mechanisms that determine affordable
access to medicines.
2.103
Medicines Australia stated that '[r]are disease molecules are often not
well-accommodated by the current processes',[143]
and opined that 'improved access to medicines via the PBS is the best way
forward'.[144]
Medicines Australia further suggested that:
As the national therapeutic goods regulatory reform agenda
has resulted in welcome amendments to the definition of such things as ‘orphan’
drugs, and will speed up regulatory approvals in certain cases of high unmet
need, it is now also time to review the reimbursement processes for those
medicines.[145]
2.104
However, Professor Andrew Wilson, Chair of the PBAC, informed the
committee that an 'orphan drug' is not a PBAC designation, but one made by the TGA,
and further noted that 'basically it's a situation where you've got a disease
where there aren't very many other treatments available for it—a rare disease
without any other treatments for it—although sometimes it's also used where
there are no other drugs'.[146]
2.105
Figure 4 sets out how the Health Technology Assessment (HTA) process—performed
by the TGA, Medical Services Advisory Committee (MSAC), PBAC and the Prostheses
Advisory Committee, which provide advice to the Australian government—works in
practice.
2.106
As can be seen, the first step in the HTA process is for a medicine to
receive regulatory approval from the TGA. This will be required for the use of
a medicine by a patient unless: a medical practitioner has been granted
authority to dispense a drug to specific patients with a medical condition; a
patient has been approved for access to a drug, which is determined on a case
by case basis; or there are specific circumstances to warrant access to the
drug.[147]
2.107
Once a drug has been approved by the TGA, a sponsor may submit an
application to the PBAC, which then determines whether a medicine will be
listed on the PBS.[148]
As Professor Wilson informed the committee, the PBAC, established pursuant to
the National Health Act 1953[149]
'to consider the effectiveness and the cost of the proposed medicine compared
with existing alternative therapies':[150]
...cannot make a positive recommendation for a medicine that is
substantially more costly than an alternative medicine unless we're satisfied
the proposed medicine also provides a significant improvement in health for at
least some population.[151]
Figure 4: Map of current
Australian Government HTA processes for market entry and for reimbursement
processes[152]
2.108
On 24 October 2014, the Australian government announced an independent
review of the regulation of medicines and medical devices (MMDR review) to:
...identify ways to assist medicine and medical device
producers and suppliers struggling with complex and costly regulatory pathways,
while upholding the safety and efficacy of therapeutic goods available in
Australia.[153]
2.109
The 58 recommendations of the review were published in July 2015, and
included:
-
expanding the pathways by which sponsors can seek marketing
approval for a medicine or medical device, including making provision for
utilisation of assessments conducted by comparable regulators, and for
expedited assessments in defined circumstances;
-
identifying comparable overseas national regulator authorities
using transparent criteria;
-
enhancing post-market monitoring of medicines and medical devices
and streamline post-market requirements in respect of products in the
Australian Register of Therapeutic Goods; and
-
improving transparency and predictability of processes and
decisions to build trust and confidence in the Australian National Regulatory
Authority's ability to ensure Australians have timely access to high quality,
safe and efficacious products.[154]
2.110
The Australian government released its response to the MMDR review on
15 September 2016, and noted that the expert panel conducting the MMDR
review:
...provided a strong case for the reform of the regulation of
therapeutic goods in Australia - one that strikes a balance between supporting
consumer choice, the safe and effective use of therapeutic products, creates
flexibility for industry and ensures that regulatory settings are appropriately
aligned to risk.[155]
2.111
The government noted its intention to implement the majority of
recommendations arising from the MMDR review:
...in a staged approach over the next three years in order to
maintain continuity of business. The Department of Health will collaborate and
consult across government and with consumers, health professionals and industry
in order to progress these reforms. The TGA, where necessary, will cost recover
from industry so as to ensure that it is adequately resourced to implement
these reforms and undertake the ongoing work without interrupting business as
usual.
The Government understands that consumer, professional, and
industry groups are looking for immediate action. Accordingly, the Department
of Health will commence work on designing implementation of the
recommendations, with a view to implementing early opportunities in 2016-2017.
Implementation of this important programme of reform will deliver significant
benefits for the Australian public and to the Australian medicine and medical
device industries.[156]
2.112
The government also recognised several benefits of its approach,
including:
-
access to life-saving and innovative medicines and medical
devices will be improved through the introduction of new, expedited pathways
for approval. This will lead to earlier access to vital, life-saving therapies
for patients with serious conditions;
-
faster access for Australian consumers to certain medicines and
medical devices that are approved based on assessments from comparable overseas
regulators. This will reduce duplication of effort, leading to efficiencies, while
ensuring Australian consumer protection is maintained through retention of
oversight by the TGA as the final decision-making authority;
-
consumer protection will be enhanced through the development of a
more comprehensive system of post-market monitoring which will provide the TGA
with better information about emerging safety issues. This will ensure that
therapeutic goods in Australia continue to be safe for use, efficacious and of
a good quality.[157]
2.113
The TGA website notes that the government has been consulting
internally, with the public, and with particular stakeholders on the
implementation of the accepted recommendations arising from the review,[158]
and states that some of the reforms 'require changes to legislation':
This large program of work was divided into two tranches; the
first set of legislative changes were passed 14 June 2017. These focused on new
assessment pathways for medicines and medical devices. The second tranche of
legislative review is underway. The progress of these amendments may influence
the timing of some regulatory changes.[159]
2.114
The reforms already implemented are:
-
those made to category C of the Special Assistance Scheme,
namely, the '[i]mplementation of a notification scheme rather than pre-approval
for supply of certain unapproved therapeutic goods to patients';[160]
and
-
the priority review pathway for prescription medicines, which
'will involve faster assessment of vital and life-saving prescription medicines
for which a complete data dossier is available' within 150 working days, which
is 'up to three months shorter than the standard prescription medicines
registration process'.[161]
2.115
As indicated above, the TGA is looking to implement a number of other
measures, such as the 'provisional approval pathway' which:
...will provide earlier access to certain promising new
medicines that do not yet have a full dossier of clinical data, but where there
is the potential for a substantial benefit to Australian patients through the
earlier availability of these medicines.[162]
2.116
In September 2015, the Senate Community Affairs References Committee (Community
Affairs Committee) reported on the effectiveness of the HTA process in respect
of the availability of new, innovative and specialist cancer drugs in
Australia.[163]
The Community Affairs Committee urged the government 'to give careful
consideration to the implementation' of the recommendations made as a result of
the MMDR review[164]
and made three key recommendations in its report, namely that the Australian
government:
-
initiate a comprehensive review of the system for the
registration and subsidisation of medicines, setting out what types of factors
should be examined;
-
commission a review of current data collection mechanisms for
cancer medicines, providing examples of factors to be included in the review;
and
-
establish a Steering Committee to examine the feasibility of
establishing a national register of cancer medicines.[165]
2.117
The government has recently responded to the Community Affairs Committee
report, in which it supported the intent of the first and second
recommendations, and did not agree to the third. In its response, the
government outlined the work it is already undertaking in response to the MMDR
review. For example, it highlighted that:
Patients and sponsors will benefit from two expedited
pathways being implemented by the TGA, which will help to achieve earlier
regulatory approvals of new life-saving medicines such as new cancer medicines,
or to extend uses of existing medicines to treat a new population of patients (for
example, a treatment already approved for one type of cancer being used to
treat another type of cancer).[166]
2.118
The government recognised that, although the MMDR review 'did not
include consideration of PBS listing and PBAC processes' the implementation
processes in response to the review will impact on these processes.[167]
2.119
The government also referred to consultation with industry that is on
foot with regard to:
... a pilot project involving a joint TGA/PBAC pre-submission
meeting, use of a single clinical evaluation report that meets both regulatory
and reimbursement authority requirements, and information sharing post-market
monitoring.[168]
2.120
Professor R John Simes advocated for further interconnectedness between
these individual mechanisms of the HTA process, namely between government
funding sources and the PBAC and MSAC. Professor Simes called for bodies such
as the MRFF to broaden their criteria for funding to include return on
investment, which he argued should also be linked to the PBAC and MSAC, as:
...if you have a drug which is supported through the PBS, there
is evidence for it. If the evidence does not exist, you cannot get funding for
that particular drug through the PBS; there is not a mechanism to do so.[169]
2.121
Further discussion about the PBAC and MSAC, and how their processes
affect LSR cancers, appears at chapter 5.
2.122
Another issue raised with the committee with respect to the HTA process is
the delay from registration by the TGA to listing on the PBS. For example,
Medicines Australia referred to its earlier submission to the Community Affairs
Committee inquiry, where it identified that this process, on average, takes 'in
excess of 18 months', and further noted:
-
New listings take on average 589
days (over 1 ½ years)
-
Subsequent listings take on
average 700 days (nearly 2 years)
-
Disturbingly, some medicines took
up to 1,600 days (4 ½ years) for a new listing and 2,400 days (more than 6 ½
years) for a subsequent listing.[170]
2.123
More
recently, Medicines Australia commissioned a Deloitte report which detailed
the duration taken in the HTA process for certain cancer medicines during the
period 2010–2016:
Table 4: Number of months
to events in the PBS process for 147 ‘high level’ submission for cancer
medicines (2010-2016)[171]
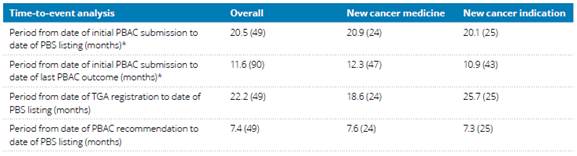
Source:
Wonder Drug Consulting, October 2016, Analysis of PBAC submissions and outcomes
for medicines for patients with cancer (2010-2016)
‘High
level’ submissions mean submissions for new medicines (i.e. new listings) and
new indications (i.e. new use within a given cancer, irrespective of PBAC major
or minor submissions.
Numbers
in parentheses are the sample sizes
2.124
Medicines Australia also provided the committee with a comparison of the
Australian reimbursement system with those of other OECD countries which
appears at Figure 5—where Australia ranks 18th out of 20 countries,
ahead of Portugal and New Zealand—also noting that 'of all the new medicines registered by the TGA
between 2009 and 2014, only 39 per cent of them were reimbursed in Australia'.[172]
Figure
5: Proportion of registered medicine which eventually secured reimbursement—by
country—2009 to 2014[173]
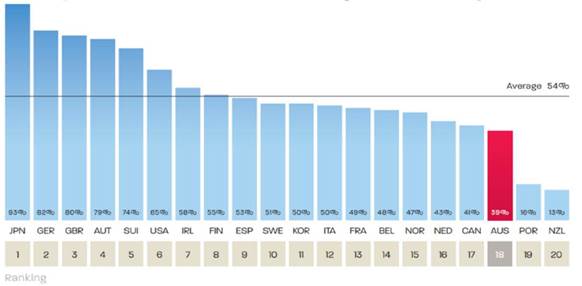
2.125
Indeed, the Community Affairs Committee outlined in its report that
a key factor that affects access to medicines,
'is the timing of applications by pharmaceutical companies to the TGA seeking registration
of medicines and to the PBAC seeking reimbursement'.[174]
Further:
The Department of Health (DOH) noted that for cancer
medicines submitted for TGA approval between 2009-2014, submissions were made
an average of 38 weeks after the lodgement of a submission to the [US] Food and
Drug Administration (FDA) and an average of 38 weeks after the lodgement of a
submission to the European Medicines Agency (EMA). DOH told the committee that
this approach is often a function of the size of the Australian market:
This kind of business approach seeks to establish, as early
as possible, a positive response in the regions offering the most potential for
profit, due to their large population size. This avoids the situation where a
deferral or rejection from a country with a small population, like Australia,
could influence other authorities, thereby jeopardising the profit margins that
could be achieved in larger countries/regions.[175]
2.126
The Community Affairs Committee acknowledged that the DoH's evidence
illustrated that 'this factor is outside the control of the TGA and PBAC', and
also cited evidence from the DoH that '[t]he ability to deliver timely access
to medicines is also affected by the timing of the applications which, in
Australia, is at the discretion of pharmaceutical companies' that may choose to
apply for approval in the US or Europe ahead of Australia.[176]
2.127
In terms of developments in the US, the committee also heard that the
FDA had recently approved, for the first time, a drug based on the molecular
profile of a tumour, rather than its location:
The FDA approved the first drug just a couple of weeks ago,
Keytruda, which is for any cancer types from anywhere in the body which is
mismatch repair deficient tumours. There is a big shift. So pharma companies
are starting to see this shift as well and look at drugs across tumour types.
From the perspective of genomics, we already think like that.[177]
2.128
Subsequently, in August 2017, the FDA made a comparable ruling on an
immunocellular therapy, which Deloitte described as 'signalling its commitment
to modernising its processes in alignment with the therapeutic landscape'.[178]
2.129
However, Professor Wilson considered that a lot of research into cell
biology is 'very basic research' that will take 'many, many years' to reach
fruition.[179]
Current funding for LSR cancers
2.130
Despite accounting for five times the number of other cancer deaths in
Australia, rare cancers receive just $6 million annually in NHMRC funding.[180]
This can be seen in Figure 6, which illustrates the total amount of funding,
including NHMRC funding, awarded to research into cancers from 2006–2011,
compared to mortality rates for these cancers.
Figure 6: National funding
to cancer type-specific research in Australia
(2006–2011) compared with the top 20 cancers by overall cancer mortality (2012)[181]
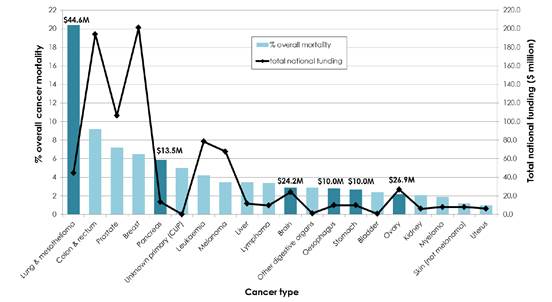
2.131
This information, and an in-depth analysis of major government and non‑government
funding of cancer research in Australia appears in Cancer Australia's 2015
publication Cancer Research in Australia: an overview of funding initiatives
to support cancer research capacity in Australia 2006 to 2011, which is the
'first national overview of funding to cancer research in Australia'.[182]
2.132
Consistent with the discussion at paragraphs 2.4–2.8 about funding into
cancer research during the period 2016–2018, Figure 7 illustrates that in
2006–2011 the Australian government was the 'major funder of cancer research projects
and research programs, people support scheme awards, and building cancer
research capacity initiatives and infrastructure awards' providing 58 per cent,
or $1.03 billion, of funding.[183]
2.133
As can be seen, 43 per cent of this funding came via the NHMRC with 15
per cent coming from other sources such as the Department of Industry
(including the Australian Research Council), Cancer Australia and the DoH.[184]
Figure 7: Proportion of
funding by funding source to cancer research projects and research programs, building
cancer research capacity initiatives, and infrastructure awards[185]
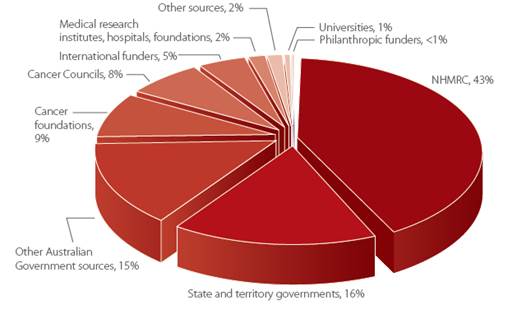
2.134
Despite this seemingly large allocation of government funding for cancer
research, the committee received a number of submissions[186]
and heard from a number of witnesses[187]
who criticised the lack of government funding for research into LSR cancers.
2.135
For example, the CBCF submitted that the government's current use of the
burden of disease approach to assess the prioritisation and funding in respect
of cancer is 'no longer an appropriate measure to use' to make this assessment,
as the use of the 'disability-adjusted life years’ (DALYs) model:
...lost appropriateness when five-year survival for higher
incidence, and comparatively well-funded, cancers (e.g. breast, prostate and
childhood leukaemia) started to get close to 100% in stark contrast to other
(far) lower-survival and (considerably) lower-funded cancers.[188]
2.136
A DALY measures the 'disease burden and combines data on the extent of
premature death and non-fatal health impacts of disease'.[189]
Using this measure as a reference for health expenditure, Cancer Australia
outlined that it was:
...estimated that in 2012, cancer caused 551,300 DALYs to be
lost, representing 19% of the burden of all diseases in Australia. By
comparison, cardiovascular disease contributed to 16% of the burden of disease,
whilst nervous system and sense organ disorders accounted for 14% of the burden
of disease and mental disorders accounted for 13% of the burden of disease. In
terms of health care expenditure, in 2008–09, cancer and other neoplasms
accounted for $5 billion or 7% of total recurrent health spending.[190]
2.137
The AIHW informed the committee that in addition to DALYs, 'quality‑adjusted
life years' (QALYs) can be used as 'a measure of potential health gain from the
effect of interventions'.[191]
Therefore, both DALYs and QALYs can 'be used in health economic evaluations as
a measure of health gain to estimate the potential health benefits of specific
health interventions'.[192]
However, the AIHW noted that the 'DALY is the standard measure used in burden
of disease studies'.[193]
2.138
Another criticism of the lack of funding into LSR cancers was raised by Ms Elizabeth
de Somer of Medicines Australia, who commented that although there had been
some welcome steps, including the announcement of the first MRFF disbursements,
'there is nothing that particularly targets the rare and low‑survival
cancers'.[194]
2.139
Indeed, the CBCF stated in its submission that LSR cancers, including
brain cancer, 'have been for some time, in effect discriminated against, within
the Government funding system'.[195]
The CBCF submitted that LSR cancers 'are clearly unmet medical needs which
should be afforded special status by earmarking specific funds and prioritising
focus around them'.[196]
2.140
Mrs Evangeline Lim, diagnosed with advanced lung cancer in
November 2016, described the personal impact of this lack of funding:
I am sad with the injustice of research funding allocated to
lung cancer. We only get less than five cents in cancer research funding, and
lung cancer has a 15 per cent survival rate of living for five years from
diagnosis.[197]
2.141
Following the due date for submissions and before the committee's final
hearing, on 24 August 2017, the government announced $13 million of
funding for competitive research grants from the MRFF, 'designed to boost
clinical trial registry activity with priority given to under-researched health
priorities, such as rare cancers and rare diseases'.[198]
2.142
The desired outcomes of this investment are as follows:
-
New opportunities for those
suffering from rare cancers and rare diseases to participate and benefit from
the latest research.
-
Attention given to under
researched health priorities and conditions.
-
Deployment of innovative trial
designs and recruitment strategies.
-
Purposeful health service engagement
to improve the translation of research into practice and improve outcomes for
patients.
-
New health treatments, drugs and
devices to improve health.
-
Reinforcement of Australia’s
position as a preferred destination for clinical trials.[199]
2.143
The DoH subsequently provided information to the committee that, from
2013–14 to 2016–17 it provided approximately $9.1 billion for cancer services
and research, which is exclusive of funding from portfolio agencies, such as
the NHMRC and Cancer Australia.[200]
2.144
In evidence to the committee on 29 August 2017, the DoH identified
several of the MRFF programs that are underway under the trials and translation
platform:
Lifting clinical trials and registries capacity, clinical
trials networks, has $5 million allocated to it. Trial activities specifically
targeting adolescents and young adults living with cancer has $5 million of
funding for CanTeen. Lifting clinical trials and registries capacity research
grants has $13 million, which is designed to accommodate clinical trials on
rare cancers and rare diseases. Eight million dollars has been allocated to the
next generation of clinical researchers.[201]
2.145
The DoH also informed the committee of MRFF investments that are
specifically relevant to rare cancers:
In the first disbursements under the MRFF, which were
announced in the context of the 2017-18 budget, $69.5 million was dispersed
from the fund. There are a couple of relevant initiatives, particularly related
to clinical trials. One is an investment in clinical trial networks, which are
often perceived to be the backbone of the trial industry in Australia. They
support investigator-driven activity. They answer questions of service delivery
and comparative effectiveness. And we have funded $5 million—the Australian
Clinical Trial Alliance— to lift the capacity of these networks that occur
across a number of specialties. That's in the process of being ramped up
We also invested $5 million through CanTeen to target trial
activity for adolescents and young adults. This cohort sometimes has difficulty
gaining access to trials—caught between kids and adults. That activity has been
executed. CanTeen is progressing with that work. Last Thursday, 24 August, the
minister announced the opening of a $13 million clinical trial and registry
program. It's actually titled Lifting Clinical Trials and Registries Capacity.
This is directly relevant to the committee because it is designed to attract
activity that addresses burden and unmet need. By that I mean rare cancers and
rare diseases. In fact, the guidelines preference rare cancer and rare disease
applicants. It also is looking at innovative trial methodologies, like, for
example, adaptive trial platforms, some innovative and novel approaches to
doing trial activity and the application of precision medicine in a trial
environment, which is increasingly being used to do a sequence of an individual
and specifically target the treatment to that patient. For lots of different
reasons, it is beneficial and, perhaps some would argue, even cost effective.
Then of course, there is investment in researchers, because
you can't just inject a whole bunch of money into the system without building
the capacity of researchers. So $8 million to top up existing NHMRC medical
practitioner fellowships—and that's progressing quite well as well too. So I
think those programs are a demonstration of the sorts of things that you may
see over time from the MRFF.[202]
2.146
The DoH also highlighted a number of features in its Medical Research
Future Fund - Lifting Clinical Trials and Registries Capacity (LCTRC) Grant
Guidelines that it considered relevant to the committee's terms of
reference:
The assessment criteria are slightly different to traditional
clinical trial structures, so they're divided into three sections. Forty per
cent is for significance of grant outcomes, another 40 per cent is for
scientific quality and 20 per cent is weighted for team quality and capacity. I
think that allocation of 40 per cent for significant grant outcomes presents a
lot of opportunity for researchers who, in the space of rare cancers and
low-survival cancers, may not have the track record of other researchers. What
we're hoping to do with that weighting is also to generate some innovative
ideas and design approaches to trials through this application round.[203]
2.147
The significance of the grant outcome is defined in the Guidelines,
where '[s]ignificance is the potential to increase knowledge of important
topics that achieve the outcomes of the grant opportunity', and will be
assessed by reference to a number of considerations.[204]
Quarantining funding
2.148
A number of submitters and witnesses advocated for specific funding to be
set aside for research into low survival rate cancers.[205]
2.149
In respect of quarantining NHMRC funding, Professor Kelso considered
that the NHMRC's current model of funding is appropriate, especially in light
of the priority-driven funding offered by the MRFF.[206]
2.150
This was reflected in the evidence of Associate Professor Wright, who
opined that quarantined funding 'could specifically target that preliminary
research that is required to build track record and eventually produce a
successful funding application', and suggested that such funding could come
from the MRFF:
I am suggesting that the NHMRC as it stands supports 13 per
cent of fundable research—that is very high-quality research. I have reviewed
that sort of research as part of my job as a researcher. I have reviewed other
people's grants, and I have seen grants that I think must get funded but that
do not get funded, just because there are not enough funds in the pool. It is
not because of any bias; it is just that that is the pool of money, that is how
much good research is being put forward, and that is how much preliminary work
has been done. Huge amounts of money and time have been put into those
applications, to go nowhere, or it has rolled over to next year. So it has to
be from outside the NHMRC. You cannot divide up the pie anymore. That is why,
if we have a new source such as the MRFF, I would say that is where that sort
of funding clearly has to come from, or it is an example of where it should
come from. I am just saying it should not come out of NHMRC.[207]
2.151
Dr Robert De Rose, who noted that the MRFF research parities had been
set for the next two years, suggested that the review of the MRFF priorities in
2018 would be:
...an opportunity to address the funding shortfall for cancers
with low survival rates. We cannot just repeat the consultation process that
was used last year to allocate funding for the first two years. This will
likely result in a similar outcome. A small amount of the research allocation
should be prioritised for low-survival cancers. Otherwise, the current
stakeholders will win out.[208]
2.152
In responding to the question of quarantining MRFF funding, Professor
Frazer noted that the powers to allocate funding are vested in the minister,[209]
pointing out that the AMRAB advises the minister about how to allocate funding,
but that 'he is not required to follow our advice'.[210]
Professor Frazer noted that the AMRAB had also recommended, going forward, that
'the grants given out should be longer term and larger scale project grants of
the order of five years' in order to 'allow bigger problems, if you like, and
problems which require more effort over a longer period of time for a larger
number of people to be contemplated'.[211]
Committee view
2.153
It is apparent to the committee that there is an inadequate amount of
government and non-government funding allocated towards research into LSR cancers.
2.154
The committee agrees with evidence it has received which demonstrates
that the rate of survival for people with LSR cancers will remain stagnant
until significantly more funding is allocated for research into these cancers.
2.155
The committee acknowledges the finite amount of government money
available for all forms of medical research, and therefore welcomes the
government's recent announcement of $13 million of funding for competitive
research grants from the MRFF that will prioritise 'under-researched health
priorities, such as rare cancers and rare diseases'.[212]
It also welcomes the more recent announcement, on 29 October 2017 of
the Australian Brain Cancer Mission, a $100 million collaboration of the
Australian government, the CBCF and philanthropy to defeat brain cancer.[213]
2.156
The prioritisation of rare cancers and rare diseases in the granting of
this funding suggests that the government acknowledges the importance of allocating
discrete amounts of funding in order to make progress in combatting rare
cancers and rare diseases.
2.157
However, the committee considers that, in order to effectively increase
survival rates for people with LSR cancers, the government should go further
and, as some submitters and witnesses have suggested, guarantee government
funding specifically for research into LSR cancers.
2.158
The committee acknowledges that the NHMRC Act prohibits the minister
from recommending 'the allocation of research funds to a particular person,
organisation, State or Territory';[214]
however, the Act also empowers the CEO of the NHMRC to identify National Health
Priority Areas (NHPAs): major national health issues that make a significant
contribution to the burden of disease[215]
to which a 'substantial proportion of NHMRC funding is directed'.[216]
'Cancer control' is one of the NHPAs in the NHMRC's Corporate Plan 2017–18.[217]
2.159
The committee urges the CEO of the NHMRC to consider identifying LSR
cancers as a NHPA in the upcoming 2018–19 Corporate Plan. The minister may be
able to require the NHMRC to do so by way of a referral, pursuant to section 5D
of the NHMRC Act, or a ministerial direction, pursuant to section 5E of the
NHMRC Act.
Recommendation 1
2.160
The committee recommends that the Chief Executive Officer of the National
Health and Medical Research Council considers identifying low survival rate
cancers as a National Health Priority Area in the upcoming 2018-19 Corporate
Plan.
2.161
The committee welcomes NHMRC's recent restructure of its grants program.
In particular, it supports the introduction of the Ideas Grant scheme which
will encourage innovation and assist early-career researchers launch their
careers. The committee considers that it is important to encourage researchers
to work on LSR cancers as this will also contribute to increased survival rates
for people with these cancers.
2.162
Further, the committee considers that the extension of the duration of
NHMRC grants—to five years for the duration of the Investigator Grants and Synergy
Grants and up to five years for the Ideas Grants—demonstrates the NHMRC's
understanding of the long time required to conduct medical research and obtain
meaningful results.
2.163
However, the committee is disturbed by the evidence that some drugs may
take 10 to 15 years to develop—much longer than a 5 year grant— and that some
research is abandoned when funding is no longer available. For these reasons,
the committee recommends that the NHMRC introduces the option for extensions to
the duration of grants, provided that recipients satisfy certain performance
criteria.
Recommendation 2
2.164
The committee recommends that the National Health and Medical Research
Council introduces the option for extensions to the duration of funding to recipients
of research grants, provided that these recipients satisfy certain performance
criteria.
Navigation: Previous Page | Contents | Next Page