Bills Digest no. 3 2015–16
PDF version [748KB]
WARNING: This Digest was prepared for debate. It reflects the legislation as introduced and does not canvass subsequent amendments. This Digest does not have any official legal status. Other sources should be consulted to determine the subsequent official status of the Bill.
Jaan Murphy, Law and Bills Digest Section
Dr David Brett, Science, Technology, Environment and Resources Section
10 August 2015
Contents
Purpose of the Bills
Structure of
the Bills
Background
Committee
consideration
Policy
position of non-government parties/independents
Position of
major interest groups
Financial
implications
Statement of
Compatibility with Human Rights
Key issues
and provisions of the Bill
Other
provisions
Key issues
and provisions of the Consequential Bill
Concluding
comments
Date introduced: 27
May 2015
House: House of
Representatives
Portfolio: Finance
Commencement: The
operative provisions of the Medical Research Future Fund Bill 2015 will
commence on the later of the day after it receives the Royal Assent or the day
after the Medical Research Future Fund (Consequential Amendments) Bill 2015
receives the Royal Assent. The schedules of the Medical Research Future Fund
(Consequential Amendments) Bill 2015 commence on the days listed in the table at item 2 of that Bill.
Links: The links to the Bills,
their Explanatory Memoranda and second reading speeches can be found on the
Bills’ home pages for the Medical
Research Future Fund Bill 2015 and the Medical
Research Future Fund (Consequential Amendments) Bill 2015 or through the Australian
Parliament website.
When Bills have been passed and have received Royal Assent, they
become Acts, which can be found at the ComLaw
website.
List of abbreviations
|
Abbreviation
|
Definition
|
|
Advisory Board
|
Australian Medical Research Advisory Board
|
|
Bill
|
Medical Research Future Fund Bill 2015
|
|
CEO
|
Chief Executive Officer
|
|
Consequential Bill
|
Medical Research Future Fund (Consequential Amendments)
Bill 2015
|
|
FFB
|
Future Fund Board
|
|
Fund
|
Medical Research Future Fund
|
|
Health Account
|
Medical Research Future Fund Health Special Account
|
|
HHF
|
Health and Hospitals Fund
|
|
Inquiry
|
Senate Community Affairs Legislation Committee inquiry
into the Bill
|
|
MAD
|
maximum annual distribution
|
|
MREA
|
Medical Research Endowment Account
|
|
MRFF Special Account
|
Medical Research Future Fund Special Account
|
|
NHMRC
|
National Health and Medical Research Council
|
|
NHMRC Act
|
National Health and Medical Research Council Act 1992
|
|
Priorities
|
Australian Medical Research and Innovation Priorities
|
|
Strategy
|
Australian Medical Research and Innovation Strategy
|
The purpose of the Medical Research Future Fund Bill 2015 (the
Bill) is to establish a perpetual fund that is capable of generating ‘income
over the long term’ to provide the ‘large-scale funding’ that is required for
‘medical research and medical innovation to support a healthy and productive
nation’.[1]
The purpose of the Medical Research Future Fund
(Consequential Amendments) Bill 2015 (the Consequential Bill) is to make
consequential amendments to other legislation to give effect to the Medical Research
Future Fund and the abolition of the Health and Hospitals Fund (HHF).
The Bill is divided into six parts:
- Part
1 contains various preliminary provisions including definitions,
extraterritorial operation and the constitutional basis for the Bill
- Part
2 establishes the Medical Research Future Fund (the Fund), defines its
purpose and sets out how payments can be made to different types of entities
- Part
3 deals with the maximum annual distribution (MAD) that can be made each
year
- Part
4 deals with how the Fund is to be invested, as well as various governance
mechanisms
- Part
5 deals with reporting obligations
- Part
6 contains miscellaneous provisions, including a rule-making power.
The Consequential Bill has three schedules. Schedule 1
contains the main amendments to a number of Acts. Schedule 2 contains
amendments relating to the abolition of the HHF. Schedule 3 contains
amendments contingent on commencement of parts of the Acts and Instruments
(Framework Reform) Act 2015.[2]
As part of the 2014–15 Budget, the Australian Government
announced the establishment of the $20 billion Fund, initially targeted to
commence from 1 January 2015.[3]
At the time, it was proposed that:
From 2015-16,
the net earnings from the Fund will serve as a permanent revenue stream, primarily
to the National Health and Medical Research Council (NHMRC). The Fund will
distribute around $1 billion a year into medical research from 2022-23... This investment, to be managed by
the Future Fund Board of Guardians, will help to ensure Australia can continue
to advance world leading medical research projects, attract and retain first
class researchers and deliver improved health and medical outcomes for all
Australians.[4]
[emphasis added]
The policy basis for creating the Fund is, in part, that by
financially supporting medical research and medical innovation over the
long-term, the health and wellbeing of all Australians can be improved.[5]
Even after Government amendments in the House of Representatives, the
mechanisms in the Bill for delivering on that policy objective differ slightly
from the original model proposed as part of the 2014–15 Budget. Nonetheless,
they are consistent with the overall policy objectives: funding medical
research and innovation (including in health disciplines) over the long-term to
improve the health and well-being of Australians.
The Parliamentary Joint Committee on Human Rights considered
the Bills and stated that they ‘do not require additional comment as they
either do not engage human rights or engage rights (but do not promote or limit
rights)’.[6]
Senate Community Affairs Legislation Committee
The Bill has been referred to the Senate Community Affairs
Legislation Committee for inquiry and report by 10 August 2015. Details of
the inquiry are at the inquiry
webpage.[7]
The Senate Standing Committee for the Scrutiny of Bills had
no comment on either the Bill or the Consequential Bill.[8]
Senate Standing Committee for the Selection of Bills
In its sixth report of 2015, the Senate Standing Committee
for the Selection of Bills (the Selection Committee) ‘considered ... but was
unable to reach agreement’ on the Bill and Consequential Bill.[9]
Subsequently however, the Senate passed an amendment to the motion to adopt the
sixth report of the Selection Committee, the effect of which was to refer the
Bill and Consequential Bill to the Senate Community Affairs Legislation
Committee for inquiry and report by 10 August 2015.[10]
Australian Labor Party
The Labor Party indicated it would not support the Bill in
its original form,[11]
and also indicated it does not support the proposed transfer of $1 billion from
the HHF to the Fund.[12]
The Labor Party has also expressed a number of concerns about the Bill related
to governance of the Fund and oversight of distributions, as well as the Bill’s
current definition of ‘medical research’.[13]
The Shadow Minister for Health, Ms Catherine King, moved a
number of proposed amendments to the Bill.[14]
Briefly, those amendments seek to ensure that decisions to provide funds,
grants or payments are made by the Chief Executive Officer (CEO) of the
National Health and Medical Research Council (NHMRC) instead of the Finance or
Health Minister. However, following the amendment of the Bill by the Government
(the details of which are discussed later in this Digest), the Labor Party
noted that:
We will not oppose these amendments, although we see them as
second-class. We think the amendments that we moved and failed to pass in the
House, which would see these funds go through the Medical Research Endowment
Account and therefore be distributed according to normal NHMRC processes, are
by [far] and away the better alternative, but we recognise that we did not have
the numbers in the House to get those through ... We will not oppose the
amendments in this House but, as the Minister knows, a Senate inquiry has just
commenced. I am advised that we will have some public hearings over the course
of July where these issues will be able to be fleshed out a little bit more,
and they will then be subject to further debate in the Senate.[15]
This would appear to indicate that the Labor party will
not necessarily support the amended Bill in the Senate.
Palmer United Party
Palmer United Party (PUP) Senator Zhenya Wang has indicated
strong support for the Fund, stating that:
The MRFF [Medical Research Future Fund] is an imperative for
Australia and that is why I and many of my Senate colleagues support it, and so
should all Australians. I call on the government to consult widely and come up
with the formula to fund it.[16]
As such, it would appear likely that the PUP will support
the Bill.
Australian Greens
The party has previously opposed linking funding for the
Fund to the previously proposed GP co-payment, but has indicated that it is
generally supportive of increasing funding for medical research.[17]
Initial funding of the Fund is linked to both savings measures in the Health
portfolio and the transfer of the uncommitted balance of the HHF.[18]
As such, the likely position of the Greens on the amended Bill is not clear.
Senator Leyonhjelm
Media reports indicate that Liberal Democrat Senator David
Leyonhjelm is opposed to the establishment of the Fund.[19]
As such, it would appear likely that Senator Leyonhjelm will oppose the Bill.
Other independents
At the time of writing, the position of other independent
Members and Senators and non-government parties was not clear.
A range of interest groups including universities, private
industry, consumer groups and industry bodies lodged more than 50 submissions
to the Senate Community Affairs Legislation Committee inquiry into the Bill
(the Inquiry).[20]
Whilst the submissions were overwhelmingly supportive of the establishment of
the Fund, a number of common issues were identified, including:
- the
expertise of Advisory Board members and how they are appointed
- the
need for consumer representation on the Advisory Board
- how
the Fund and NHMRC would coordinate efforts
- ensuring
the Strategy and Priorities are developed using evidence and are not politicised
- the
need to support indirect research costs
- the
definitions of ‘medical research’ and ‘medical innovation’
- how
funding decisions are made and the role of peer-review and independent expert
advice
- the
need for the Investment Mandate to focus on or prohibit certain types of
investments
- reporting
obligations and
- the
need for the Advisory Board.
These issues are examined below under the heading ‘Key issues and provisions of the Bill’.
In the second reading speech for the Bill, the Treasurer
advised that on it establishment on 1 August 2015:
The fund will then receive an initial
contribution of $1 billion from the uncommitted balance of the Health and
Hospitals Fund. In addition, the estimated value of savings from the Health
portfolio will be contributed until the fund breaches a target capital level of
$20 billion.[21]
The Financial Impact Statement in the Explanatory
Memorandum to the Bill notes that:
- the
initial credit of funds from the HHF into the Fund will not have a direct
impact on underlying cash and fiscal balances
- interest
earnings of the Fund will have a positive impact on the underlying cash and
fiscal balances
- costs
incurred by the Future Fund Board (FFB) will have a negative impact on the
underlying cash and fiscal balances and
- payments
from the Fund will have a negative impact on the underlying cash and fiscal
balances.[22]
However, the Financial Impact Statement does not provide
any figures regarding the financial implication of the Bill over the long term
arising from the impacts noted above. The way in which the establishment of the
fund was accounted for in the budget has attracted some criticism, with one
commentator stating:
Of course, since the government is in deficit, it doesn't
actually have any money to put into its medical research future fund account.
So to its normal borrowing to cover the deficit it will have to add borrowing
to finance the money it puts into the research fund. This extra will add to the
size of its gross public debt, but not to its net debt, since the
latter is the gross debt (everything the government owes other people) minus
all the money in the various parts of the future fund, which has been used to
buy shares and bonds, and so represents all the money other people owe the
government. However, when the government spends the interest on the medical
fund on medical research, this spending will be recorded above the line and so
will add to the deficit.[23]
As required under Part 3 of the Human Rights
(Parliamentary Scrutiny) Act 2011 (Cth), the Government has assessed the
Bills’ compatibility with the human rights and freedoms recognised or declared
in the international instruments listed in section 3 of that Act. The
Government considers that the Bills are compatible.[24]
In the House of Representatives, the Government moved a
number of amendments to the Bill and Consequential Bill.[25]
The amendments appear to be a response to criticisms regarding the governance
of the Fund and oversight of distributions, with the Minister stating:
These amendments clarify and enhance the decision-making and
accountability mechanisms to be used in the disbursement of funds from the
MRFF. They reflect the policy approach already announced, but for public
assurance the government is very willing to insert arrangements into the
legislation that were originally intended to be implemented administratively.
By shifting this detail into the legislation, the government can disabuse
concerns raised by the opposition that disbursements from the fund may lack
sufficient governance or expert leadership. The amendments ensure robust
decision making and strong accountability mechanisms.[26]
As a result, the analysis and discussion below refers to
the Bill as amended by the House of Representatives. Briefly the amendments:
- create
the Australian Medical Research Advisory Board (Advisory Board)
- impose
requirements to have:
-
an
Australian Medical Research and Innovation Strategy (Strategy) and
- Australian
Medical Research and Innovation Priorities (Priorities)
both
of which must be developed by the Advisory Board and
- specify
that decision-making mechanisms for the disbursement of funds from the Fund must
take account of the Strategy and the Priorities.
Part 1 – preliminary matters
Objects of the Bill
The Bill is somewhat unusual in that it contains both an
objects clause (clause 3) and a preamble. The preamble provides that:
The Parliament of Australia recognises that the health and
wellbeing of all Australians is essential to the future of Australia.
Discoveries in medical research and important medical innovations in the future
will contribute to improving the health and wellbeing of all Australians.
In order for medical research and medical innovation to
support a healthy and productive nation, long-term and large-scale funding is
required. The Parliament believes that the establishment of a perpetual fund
capable of generating income over the long term is the most appropriate
mechanism for ensuring that this funding is available on an ongoing basis. The
Commonwealth has a role in meeting this funding need as it is able to marshal
and deploy resources not available through other means.
Funding a system for medical research and medical innovation
requires a national approach. The establishment of the Medical Research Future
Fund and its administration will ensure that a coherent and consistent approach
is adopted in the funding of medical research and medical innovation to ensure
that such research and innovation benefits all Australians.
Clause 3 provides that the object of the Bill is
to:
... improve the health and wellbeing of Australians by
establishing the Medical Research Future Fund to provide grants of financial
assistance to support medical research and medical innovation.
The contents of the preamble and clause 3 are both
broadly consistent with the policy objective contained in the Bill’s
Explanatory Memorandum and that proposed as part of the 2014-15 Budget.
Constitutional basis of the Bill
Clause 9 of the Bill provides a number of
‘alternative’ constitutional bases for the validity of the Bill.[27]
The inclusion of such a clause would appear to be a reaction to the High Court
decisions in Pape, Williams (No. 1) and Williams (No. 2).[28]
In simple terms, the High Court has held that even if the Commonwealth has a
valid constitutional basis for spending money, specific legislative
authorisation beyond an appropriation Act is usually required before it
can do so.
Until the High Court’s 2009 decision in Pape, the
prevailing view was that the Commonwealth had the power to appropriate monies
to a purpose or matter irrespective of whether or not the Commonwealth had any
legislative power in relation to that purpose or matter. This purported ‘spending
power’ was inferred from sections 81 and 83 of the Constitution, which
deal with appropriations.
However, in Pape the High Court ruled that sections
81 and 83 of the Constitution do not of themselves provide the
Executive Branch of the Commonwealth Government with a substantive spending
power. Instead, the Commonwealth has to show some legal basis,
beyond the mere passing of an Appropriation Act, for expenditure to be
lawful. Put simply, spending can only usually be authorised by
legislation that falls within a subject matter head of power under section 51
of the Constitution or by the ‘nationhood power’.
In Williams (No. 1) the High Court again held that
the Executive Branch of Commonwealth Government cannot (generally) spend money
unless it is supported by constitutionally valid, legislatively provided power.
Hayne J noted pointedly that the proposition that just ‘because certain
expenditure could be authorised by statute, it can be undertaken by the
Executive’ was false.[29]
Put simply, even if the Constitution provides a head of power that could
support legislation that could then authorise certain expenditure (in
this case, on medical research) that doesn’t mean the Government can
spend money prior to such legislation being passed. The Commonwealth
Parliament passed what it perceived as remedial legislation to counteract the Williams
(No. 1) decision.[30]
The validity of that legislation was the focus of Williams (No. 2).
In Williams (No. 2) the High Court held that the
remedial legislation was also not authorised under the incidental power (section
51(xxxix) of the Constitution) because it would allow any appropriations
made under sections 81 and 83 of the Constitution to be spent as the
Executive branch of the Commonwealth Government saw fit, against the authority
of Pape. Put simply, once again the High Court held that all
expenditures must be authorised by legislation supported by a relevant
constitutional power — it is not simply enough to pass an Appropriation Act.
Clause 9 appears to be a reaction to these High
Court decisions and outlines fourteen constitutional heads of power that
authorise ‘making grants of financial assistance to support medical research
and medical innovation’[31]
including:
- the
grants power (section 96 of the Constitution)
- the
health and social services power (section 51(xxiiiA) of the Constitution)
- the
territories power (section 122 of the Constitution)
- the
corporations power (section 51(xx) of the Constitution) and
- the
race power (section 51(xxvi) of the Constitution).[32]
Whilst the inclusion of clause 9 does not guarantee
that aspects of the Bill will not be susceptible to some form of constitutional
challenge in the future, it aims to ensure that other provisions in the Bill remain
valid in the event of such a challenge being successful.
Key definitions
Clause 5 contains key definitions that underpin the
operation of the Bill, some of which are examined below.
Advisory board
The definition of Advisory Board means the Australian
Medical Research Advisory Board proposed by clause 32B.
Australian Medical Research and Innovation Priorities
Australian Medical Research and Innovation Priorities
(Priorities) is defined to mean the priorities determined by the Advisory
Board in accordance with clause 32E.
Australian Medical Research and Innovation Strategy
Australian Medical Research and Innovation Strategy
(Strategy) is defined to mean the strategy determined by the Advisory Board
in accordance with clause 32D.
Business entity
The definition of business entity is broad and
encompasses most types of entities used to undertake business activities (for
example, companies and trusts), other than cooperatives.
A number of submissions either supported or were critical of
the definition of medical research proposed by the Bill.[33]
Medical research is only defined as including ‘research into health’. Compared
to the definition contained in the National Health and Medical Research
Council Act 1992 (NHMRC Act, discussed below), this is a vague
definition that could be taken to allow the funding of research into
human/animal/plant health, or possibly into broader health determinants such as
agricultural research (for example, that relates to nutrition) or socioeconomic
or sociocultural factors that influence health. In contrast, section 4 the NHMRC
Act defines medical research as including (but not limited to):
... the laboratory-based or clinical study, or group or
community-based study of the causes, treatment and prevention of human diseases
and also includes dental research.[34]
The lack of a precise definition of medical research has
raised some concerns, with the Shadow Minister for Health noting that:
Departmental officials at Senate estimates yesterday ... were
unable to give the Senate estimates committee even a definition of 'medical
research' that would be funded under the scheme. Instead, we were told a yet to
be appointed ministerial advisory committee that is not in the legislation will
advise the government on what research could be funded under the program ...
[d]esigning mobile phone apps is one of the uses the fund could possibly be put
to.[35]
The Shadow Minister then moved that the ‘House notes that
the Bill ... does not define medical research and innovation in the way in which
the government has itself described it’.[36]
These concerns were echoed by some interest groups. For
example, the University of Melbourne expressed the view that the current
definition of ‘medical research’ contained in the Bill could:
... inadvertently exclude those non-medically related
disciplines such as physics, engineering, information technology and
mathematics (and also allied health disciplines) that are part of the
contemporary approach to medical research.[37]
As a result, it recommended that ‘the Bill adopts a broad,
inclusive definition of ‘medical research’ to reflect the multidisciplinary
nature of modern medical research’, and proposed that the following definition
would best facilitate coverage of contemporary best practice in medical
research:
Medical research can draw on many disciplines and is broadly
defined as the investigation into the causes, prevention and treatment of
disease that includes, but may not be limited to, an understanding of
fundamental biological processes, applications of basic research or
translational research that generate new knowledge in the fields of
biomedicine, clinical medicine, trauma, public health or allied health
sciences.[38]
The Australian Health Economics Society (AHES) also
expressed concern, arguing that ‘an inappropriately narrow medical
interpretation of research priorities could become entrenched’ unless a clear
statement of the research scope intended to be covered is incorporated into the
Bill.[39]
Likewise, the Group of Eight (Go8)[40]
argued that the Bill’s definition of ‘medical research ‘provides no specific
guidance about the range of research disciplines and approaches that the Fund
can support’ and therefore ‘is likely to hinder Australia’s capacity to produce
truly outstanding advances in health and wellbeing.’[41]
In contrast, a number of other submissions argued that the
definition of ‘medical research’ provides a sufficient level of flexibility and
should be retained.[42]
A number of submissions either supported or were critical
of the definition of medical innovation proposed by the Bill.[43]
Medical innovation is broadly defined as:
... the application and commercialisation of medical research,
and the translation of medical research into new or improved medical
treatments, for the purpose of improving the health and wellbeing of
individuals.[44]
Given that the definition links to the vague definition of
medical research discussed above, it has potentially broad application. Whilst
this may provide the Fund with significant flexibility with regards to the
provision of funding, awarding of grants and investment decisions, it also
introduces a degree of ambiguity as to precisely what activities are covered,
at least in comparison to the existing framework under the NHMRC Act.
Further, this definition would appear to allow the Fund to
support commercialisation activities that are currently ineligible for funding
through the NHMRC, such as phase 4 clinical trials, which in most cases are not
deemed to be research. Phase 4 clinical trials are studies:
... designed to monitor the effectiveness of the approved
intervention in the general population and to collect information about any
adverse effects associated with widespread use over longer periods of time.
They may also be used to investigate the potential use of the intervention in a
different condition, or in combination with other therapies.[45]
Traditionally, because phase 4 clinical trials occur after
an intervention, treatment or drug has already been approved for certain uses
and is being marketed, it has not been considered medical research of the type
that would attract funding through the NHMRC.
It is unclear how broadly the funding of commercialisation
of medical research is intended to be applied; this could include marketing,
for example, as that is (arguably) an aspect of commercialising new or improved
medical treatments.
As with the definition of ‘medical research’, a number of interest
groups expressed concern about the definition of ‘medical innovation’, with
some arguing that it requires amendment to improve clarity and ensure it can be
flexibly applied to a wide range of activities. For example, the University of
Notre Dame Australia argued that the definition is too narrow as it only
focuses on ‘medical treatments’ and that:
There are many examples of valuable research that do not
relate to medical treatment including in the areas of prevention, health risk
management and early detection of diseases and other health problems. In
researching better care for chronic disease multidisciplinary care should be a
focus rather than ‘medical treatments’ alone. And beyond treatment and cure the
important roles of palliative care research and pain management need to be
recognized in the health and medical research agenda. The delivery of quality,
accessible and efficient health services is another national priority requiring
a range of research disciplines including health services, health economics,
health policy research that changes clinical practice and informs health
policy.[46]
Accordingly, it suggested that the Bill be amended to
include alternative definition such as:
Medical innovation means the application,
commercialisation and translation of medical research and invention to create
new or better ways to improve the health and wellbeing of individuals and communities.[47]
Likewise the MRFFAG also recommended that the definition
of ‘medical innovation’ be amended to ‘include reference to diagnosis and
prevention’ and proposed an alternative definition very similar to the one
proposed by the University of Notre Dame Australia.[48]
However, other submissions argued that the definition of ‘medical innovation’ provides
a sufficient level of flexibility and should be retained.[49]
Medical research institute
A medical research institute is defined as an
institute (however described) that:
- has
a primary purpose of conducting medical research and
- is
an entity that is registered under the Australian Charities and
Not-for-profits Commission Act 2012 as the type of entity mentioned in
column 1 of item 1 of the table in subsection 25-5(5) of that Act.[50]
Corporate Commonwealth entity
A corporate Commonwealth entity is defined as having the
same meaning as in the Public Governance, Performance and Accountability Act
2013.[51]
That Act provides for two types of Commonwealth entities–those that are body
corporates (such as the Commonwealth Scientific and Industrial Research
Organisation (CSIRO)) and those that are not (such as all the Commonwealth
departments, including the Department of Health). A corporate Commonwealth
entity ‘has a separate legal personality and can act on its own behalf in
exercising certain legal rights such as entering contracts and owning property’
in contrast, non-corporate Commonwealth entities ‘have no separate legal
existence from the Commonwealth’.[52].
Crown immunity from prosecution for
offences
Subclause 6(1) provides that the Act will bind the
Crown in each of its capacities. However, subclause 6(2) then provides
that the Act will ‘not make the Crown liable to be prosecuted for an offence’,
which in effect, provides a limited form of Crown immunity.
Crown immunity is defined as ‘the presumption that a statute
does not bind the Crown unless by express mention or necessary implication’ (in
this instance, subclause 6(1) does so explicitly).[53]
Importantly, the ‘Crown’ has been interpreted as ‘often signifying the entire
administrative edifice of the executive government’[54]
and hence arguably extends to public servants acting in their official
capacities. One of the immunities enjoyed by the Crown includes immunity from
prosecution of a criminal offence (at common law or under statute) which is
what is specifically granted by subclause 6(2).[55]
Whilst providing immunity from prosecution is not unheard
of, the Explanatory Memorandum and the second reading speech do not provide any
explanation as to why this immunity is required in this instance.[56]
Extraterritorial application
Clauses 7 and 8 provide that the operation of
the Bill will extend to every external territory of the Commonwealth, and also to
acts, omissions, matters and things outside Australia. The Explanatory
Memorandum to the Bill explains that it is necessary for the legislation to
apply outside Australia because grant recipients ‘may collaborate with
international partners’.[57]
Part 2 – The Fund
Part 2 of the Bill is divided into five Divisions:
- Division
1 provides a simplified outline of Part 2
- Division
2 provides for the establishment of the Fund and the Medical Research
Future Fund Special Account (MRFF Special Account)
- Division
3 deals with credits of amounts to the MRFF Special Account
- Division
4 has six subdivisions dealing with the limits on total annual debits from
the MRFF Special Account, the purpose of the MRFF Special Account, making
grants and payments and certain obligations of the FFB
- Division
5 deals with transfers from the Fund to the Future Fund.
Key issues and provisions in each Division are examined
below.
Division 1 — Introduction
Clause 10 contains a simplified outline of Part 2 of the
Bill. It notes that:
- the
Fund consists of the MRFF Special Account and the investments of the Fund
(Investment Portfolio)[58]
- initially,
the Fund’s investments are a portion of the investments of the HHF which was
established under the Nation-building Funds Act 2008 [59]
- debits
are made from the MRFF Special Account by the Finance Minister after
being required to do so by the Health Minister[60]
- the
Health Minister takes the Priorities (determined by the Advisory Board) into
account in making decisions about the financial assistance provided from the MRFF
Special Account[61]
- the
MRFF Special Account can be debited for three main purposes:[62]
- channelling
grants to the COAG Reform Fund to make grants of financial assistance to States
and Territories[63]
- channelling
grants to the Medical Research Future Fund Health Special Account (Health Account)
to make grants of financial assistance to certain bodies[64]
- making
grants of financial assistance directly to corporate Commonwealth entities[65]
and
- the
MRFF Special Account can also be debited in relation to costs and other
obligations incurred by the FFB in managing the Fund.[66]
Division 2 — Establishment of the
Fund and Special Account
Clause 11 establishes the Fund, and provides that it consists
of the MRFF Special Account and the Investment Portfolio. Clause 14
establishes the MRFF Special Account, which will be a special account for the
purposes of the Public Governance, Performance and Accountability Act 2013.
In turn, an Appropriation Act can contain a provision to the effect that
if any of the purposes of the MRFF Special Account is a purpose covered by an
item in an Appropriation Act (even if it doesn’t expressly refer to the MRFF
Special Account) then those amounts may be debited against the appropriation
for that item, and credited to that special account without further legislative
authorisation.[67]
Initial seed capital and investments
Clauses 12 and 13 give effect to the policy basis
for providing the Fund’s initial seed capital and investments announced in the 2014–15
Budget.[68]
Clause 12 provides that the day after the section commences, the Finance
Minister must determine the amount to be transferred from the HHF to the Fund,
and give the determination to the FFB.[69]
The determination will be a non-disallowable legislative instrument.[70]
In turn, clause 13 provides that within 28 days of
receiving the determination the FFB must identify financial assets (to the
value of the amount determined by the Finance Minister) to be transferred from
the HHF to the Fund. Further, paragraph 13(1)(b) provides that the FFB
must also ensure that the balance of the HHF not transferred to the Fund will
stand to the credit of the HHF Special Account.[71]
Division 3 — Credits to the MRFF
Special Account
Clause 15 allows the responsible Ministers[72]
(or their delegates) to determine an amount that is to be credited to the MRFF
Special Account.[73]
Any such determination will be a non-disallowable legislative instrument, with the
Government noting that:
Such determinations would usually be regarded as administrative,
rather than legislative, in character. It is not appropriate that they be
disallowable as they are a one-off instrument made when funds are about to be
credited. The same approach was taken for equivalent provisions in the Future
Fund Act 2006.[74]
In effect, clause 15 allows amounts other than those
included in the initial transfer of assets from the HFF to be credited to the
MRFF Special Account. Subclause 15(2) provides that when making the
written determination, the responsible Ministers must ‘have regard to the
object of this Act’, which is set out in clause 3. These are discussed
below under the heading ‘Objects of the Investment Portfolio’.
Division 4
— Debits from the MRFF
This division is divided into six subdivisions. Subdivision
A deals with the rules that apply to debits from the MRFF Special Account,
including the limit on total annual debits. The majority of the other
subdivisions deal with the powers and process involved in making grants to the
states, territories and various corporate entities (such as universities,
companies and Commonwealth owned corporations). It is this aspect of the Bill (as
drafted) that has attracted controversy, as the proposed mechanisms for
determining successful grant applicants and then awarding grants differs from
the existing framework that governs the awarding of Commonwealth grants for
medical research created by the NHMRC Act. These differences remain, and
are discussed below.
A large number of submissions expressed concern over the
how individual funding decisions were to be made, the decision making process
and the role of the Advisory Board and the Minister. These submissions
expressed a strong preference that funding decisions be made either on the
basis of independent expert advice and/or through a peer review process,
instead of being determined by the Minister and subject to Cabinet approval
procedure.[75]
Originally, the Bill contained no information on how
decisions on grant funding are to be made. In contrast, the NHMRC Act provides
that a function of the CEO of the NMHRC is to provide advice to the Minister on
a range of issues related to medical research, including making recommendations
in relation to expenditure on medical research (including recommendations in
relation to making of grants for medical research).[76]
Currently, subsection 51(2) of the NHMRC Act provides
that grants for medical research must be provided ‘subject to such conditions
as the Minister, acting on the advice of the CEO, determines’ (emphasis
added). In other words, the current framework for awarding medical research
grants requires the Minister to ‘act on’ the advice provided by an independent
statutory authority (the CEO of the NHMRC). The Explanatory Memorandum
indicates that the government intends:
... to rely on the expertise of the NHMRC to aid the
distribution of funds from the Medical Research Future Fund for the purpose of
making grants of financial assistance to support medical research and medical
innovation.[77]
Originally the Bill lacked similar provisions, and appeared
to provide that grants from the MRFF Special Account could be made at a
Minister’s discretion and without a legal requirement for the Minister to at
least consider independent, expert advice from a statutory office holder or to consult
with the NHMRC. The Opposition raised concerns about those differences,
stating:
There is no peer review and no independent oversight in the
legislation at all, which will allow the government to fund its own pet health
and medical research projects... the reason that Labor has such significant
concerns is that this Bill... does not provide the governance assurances that
would satisfy Labor that the fund credits will be disbursed in the most prudent
manner. Frankly, the way that the government has established it leaves no
assurance that funds will not simply be channelled to fund the coalition's own
election commitments and pet projects, so long as they meet very broad purposes
as stated in the Bill.[78]
In an apparent response to these criticisms, the
Government made a number of amendments to the Bill, which the Minister stated:
... increase transparency in the decision-making process
[to] include the requirements for an expert Australian medical research
advisory board, setting out the advisory board's role, governance and reporting
arrangements; an Australian medical research and innovation strategy;
Australian medical research and innovation priorities; and decision-making
mechanisms for the disbursement of funds from the MRFF to be reliant upon the
strategy and the priorities.[79]
[emphasis added].
Clause 15A was a Government amendment agreed to in
the House of Representatives.[80]
It provides that the Health Minister ‘must take into account’ the
Priorities when determining whether to require the Finance Minister to debit a
specified amount from the MRFF Special Account. As explained by the Minister:
The proposed amendments also clarify the finance minister's
role. The finance minister is not the decision maker... Debits from the MRFF
special account will only be made by the finance minister after being required
to do so by the health minister. The role of the finance minister merely
credits amounts between accounts as a bursar of funds, acting on instruction of
the health minister.[81]
Importantly, as noted in the Bill’s Supplementary
Explanatory Memorandum:
- the
Health Minister will bring forward proposals for Cabinet’s consideration that
are consistent with the Strategy and Priorities (these are discussed below
under the headings ‘Division 3 – the Strategy’ and ‘Division 3 – the Priorities’)
- the
Priorities must be used by the Government when ‘in making decisions regarding
programme-level funding from the MRFF’[82]
- the
application of the Priorities to inform decision-making by the Cabinet and
Health Minister is ‘expected to ensure that any expenditure from the MRFF will
have a strong business case’[83]
and
- programme-level
funding decisions are then made by Cabinet through the Budget process.[84]
As a result, whilst the Minister still retains a level of
discretion over how grants and payments are to be awarded and to what specific
programs, the requirement imposed on the Health Minister by subclause 15A(2)
to ‘take into account’ the Priorities set by the proposed Advisory Board
ensures that the Minister is provided independent expert advice regarding potential
funding decisions.[85]
The Supplementary Explanatory Memorandum succinctly summarises the grant
funding decision making process, and the role of the proposed Advisory Board in
relation to the Health Minister and Government generally in the diagram below:[86]
Figure 1: MRFF governance and disbursement arrangements
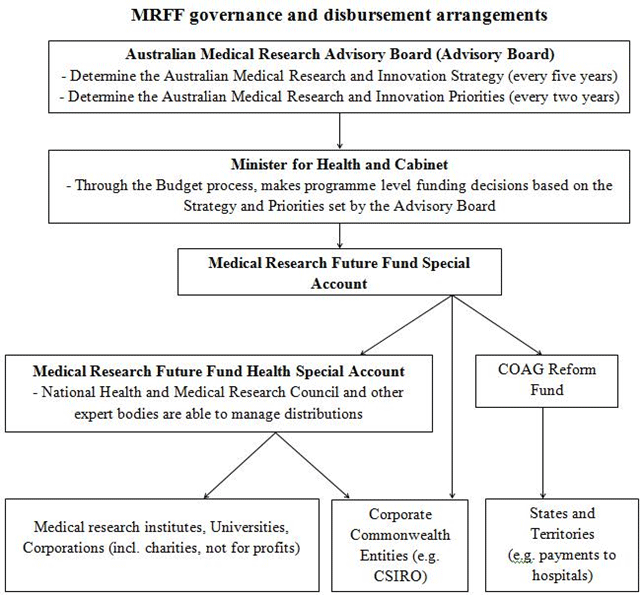
Source: as per footnote 86
However, a large number of submissions to the Senate
Community Affairs Legislation Committee Inquiry into the Bill have expressed
dissatisfaction or concern about the proposed method for making individual
funding decisions, including the role of the Minister and recommended an alternative
approach.[87]
The table below summarises those submissions.
Table 1: peer review of funding
decisions
|
Interest group
|
Recommendation
|
|
Australian Society for
Medical Research
|
- Once the research priorities are established,
research funds should be allocated following rigorous peer-review of grants
applications.
- to avoid duplication, reduce bureaucracy, cut red
tape and maximise the ‘add on value’ of the MRFF, the Advisory Board for the
MRFF should sit under the umbrella of the NHMRC Research Committee and
- the existing NHMRC grant application processes should
be utilised to ensure the best quality health and medical research is funded,
and a smooth and transparent funding mechanism for the MRFF funds.
|
|
Orygen – The National Centre
of Excellence in
Youth Mental Health
|
- The NHMRC’s capacity to provide appropriately
peer-reviewed assessment of research proposals will be a key asset that
should be used in the disbursement of a substantial proportion of funding
allocated from the MRFF.
- Expert review should form a part of decision-making
for all disbursement channels.
|
|
Australian Academy of
Technological Sciences and Engineering
|
- Funding allocations need to be competitive, assessed
by expert review and merit based.
|
|
Victorian Government
|
- The Bill should be amended to ensure that funding is
directed to medical research solely on the basis of independent and peer
reviewed advice.
|
|
Medical Research Future Fund
Action Group
|
- Public, competitive calls for applications should be
the norm with independent expert assessment of proposals for financial
assistance.
- Clauses 20,
25 and 29 should be amended to require the Minister to obtain and
consider appropriate and relevant independent expert advice in relation to a
direction that amounts are to be debited from the MRFF Special Account or the
MRFF Health Special Account.
|
|
Group of Eight
|
- Independent expert review represents international
best practice in the allocation of scarce funding, and should be included in
the selection and allocation of project funding from the MRFF.
- The Senate should amend the Bill to include the need
for independent expert review or advice as part of the process of
distributing funds from the MRFF Special Account.
|
|
The University of Melbourne
|
- The Bill should be amended to acknowledge the
centrality of independent, expert review of research applications as best
practice in the transparent, efficient allocation of competitive research
funding and require that it be adopted for allocating grants through the MRFF.
|
Source: as per footnote
87.
The need to support indirect
research costs
A number of submissions highlighted the desirability for
grants made from the MRFF Special Account to be able to fund indirect research
costs.[88]
For example, Universities Australia (UA) noted that the ‘inadequate level of
support for the indirect costs of research has long been identified as a
serious concern for the Australian research system’.[89]
The Victorian Government stated that in its view, Commonwealth-funded research
should fund the full costs of such research ‘including indirect costs’ through
a ‘consistent health and medical research grants’ program open to ‘all
administering institutions, including universities, hospitals and medical
research institutes’.[90]
In addition to not placing independent expert advice and
peer-review of funding applications at the centre of the funding
decision-making process, the Bill differs from the existing framework in other important
aspects.
General purposes of the MRFF
Special Account
Subdivision B of Division 4 of Part 2 deals
with the purposes of the MRFF Special Account. Clause 17 outlines the
three main purposes of the MRFF Special Account as per the simplified outline of
Part 2 provided in clause 10 (discussed above). Clause 18 defines
the purposes of the MRFF Special Account that are related exclusively to the
Investment Portfolio. For example, these purposes include:
- paying
the costs of, or incidental to, the acquisition of financial assets or derivatives
under clauses 37 and 47 respectively[91]
- paying
a premium in respect of a contract of insurance entered into by the FFB exclusively
in connection with the Fund[92]
and
- paying
expenses of an investment of the Fund.[93]
As such, it sets out the additional purposes (expenses
that are exclusively related to the Investment Portfolio) for which the MRFF
Special Account may be debited.[94]
In addition, clause 19 lists a number of purposes that, whilst not
exclusively related to the Fund, are nonetheless a ‘purpose’ of the MRFF
Special Account. In effect, clause 19 facilitates payment of various
expenses that may be incurred by the FFB in respect of its broader functions
under the Bill, and related legislation from the MRFF Special Account.[95]
Specific purpose of the MRFF
Special Account: State/Territory grants through the COAG Reform Fund
Subdivision C deals with providing funding to states
and territories for ‘the purposes of’ medical research and medical innovation. Clause
20 provides that the Finance Minister must, if required by the Health
Minister to do so under clause 15A, direct that a specified amount be
debited from the MRFF Special Account and credited to the COAG Reform Fund for
the purpose of providing a specific grant to a state or territory for medical
research and medical innovation. A copy of any such written direction must be
given to the Treasurer and Health Minister.[96]
Clause 22 states that the terms and conditions of
such grants must be set out in a written agreement between the Commonwealth and
state or territory, and must be complied with. Clause 21 then provides
that the Treasurer must ensure that the COAG Reform Fund is debited for the
purposes of making the grant.
The Opposition unsuccessfully moved amendments to clauses
20 and 22 which would have replaced the references to the Finance
Minister and ‘a Minister’ in those clauses with references to the CEO of the
NHMRC.[97]
The effect of the amendments would be to remove the power and discretion to determine
what grants will be provided to state and territories (and under what terms) from
the Ministers, and instead provide that power solely to the CEO of an
independent statutory authority (the NHMRC).
Specific purpose of the MRFF
Special Account: grants to corporate entities and universities
Subdivision D deals with providing grants to a range
of entities through the MRFF Health Special Account. In contrast to the grants
provided to states and territories under subclause 20(2), grants under
this subdivision do not need to be ‘for the purposes of’ medical research and
innovation but instead can be for the broader purpose of ‘supporting’ medical
research and medical innovation by:
- medical
research institutes
- universities
- corporate
Commonwealth entities and
- corporations.[98]
Clause 23 establishes the MRFF Health Special Account
(Health Account), which will be a special account for the purposes of the Public
Governance, Performance and Accountability Act 2013. In turn, an Appropriation
Act can contain a provision to the effect that if any of the purposes of
the Health Account is a purpose covered by an item in the Appropriation Act
(even if it doesn’t expressly refer to the Health Account) then those amounts
may be debited against the appropriation for that item, and credited to that
special account without further legislative authorisation.[99]
Clause 24 specifies that the purpose of the Health
Account is to ‘make grants for the purposes of supporting medical research and
medical innovation’ and that these grants can be made to the entities listed
above. As noted earlier, the use of the word ‘supporting’ widens the range of
activities that could potentially be funded under such grants, and may possibly
extend to activities that only tangentially ‘support’ medical research and
medical innovation, such as marketing.
Clause 25 provides that the Finance Minister must, if
required to do so by the Health Minister under clause 15A, direct
that a specified amount be debited from the MRFF Special Account and credited
to the Health Account for the purpose of providing a grant to an entity of the
kind referred to in clause 24. A copy of any such written direction must
be given to the Treasurer and Health Minister.[100]
Clause 26 then provides that the Health Minister must
ensure that the Health Account is debited for the purposes of making the grant.
The Government notes that this:
... reflects the Government’s intention that the MRFF Health
Special Account operates as a vehicle through which payments from the Medical
Research Future Fund will be distributed to recipients in respect of medical
research and medical innovation initiatives.[101]
Following such a debit, the Health Minister must publish
on the internet information about the grant to which the debit relates,
providing a measure of transparency (dependent of course on the level of detail
of such publications).[102]
Clause 27 states that the terms and conditions of
such grants must be set out in a written agreement between the Commonwealth and
the entity receiving the grant, which must be complied with.
Specific purpose of the MRFF
Special Account: payments to corporate Commonwealth entities
Subdivision E deals with providing funding to corporate
Commonwealth entities for ‘the purposes of supporting’ medical research and
medical innovation. Clause 29 provides that the Finance Minister must,
if required by the Health Minister to do so under clause 15A, direct
that a specified amount be debited from the MRFF Special Account for the
purpose of providing a grant to a corporate Commonwealth entity for the
‘purposes of supporting’ medical research and medical innovation. A copy of any
such written direction must be given to the Treasurer and Health Minister.[103]
As noted earlier, the use of the word ‘supporting’ widens the range of
activities that could potentially be funded under such grants.
Clause 30 states that the terms and conditions of
such grants must be set out in a written agreement between the Commonwealth and
the corporate Commonwealth entity, and those must be complied with.
The Opposition unsuccessfully moved amendments to clauses
29 and 30 which would have replaced the references to the Finance
Minister and ‘a Minister’ in those clauses with references to the CEO of the
NHMRC.[104]
The effect of the amendments would be to remove the power and discretion to
determine what grants will be provided to corporate Commonwealth entities (and
under what terms) from the Ministers, and instead provide that power solely to
the CEO of an independent statutory authority (the NHMRC).
Obligation on FFB to ensure
sufficient money
Subdivision F deals with the obligation of the FFB to
ensure that there is sufficient money in the Special Account to cover
authorised debits.
Division 5 — Inter-fund transfers
Division 5 deals with inter-fund transfers between
the Fund and the Future Fund. Clause 32 allows the Finance Minister to
direct that any amount debited from the Future Fund Special Account for the
purposes of the Bill is to be debited from the MRFF Special Account and credited
to the Future Fund Special Account. The Explanatory Memorandum notes that the
purpose of clause 32 is to allow ‘reimbursement to the Future Fund
Special Account of expenses incurred in relation to the Medical Research Future
Fund that have been debited from the Future Fund Special Account’.[105]
Part 2A was inserted into the Bill by the
Government’s amendments and is comprised of four divisions.[106]
The purpose of Part 2A is to establish the Advisory Board, the Strategy
and the Priorities and provide arrangements for the appointment of Advisory
Board members and related issues.
Division 1 — simplified outline
Clause 32A contains a simplified outline of Part
2A that outlines the role of the Advisory Board, Strategy and Priorities. The
Advisory Board:
- consists
of the CEO of the NHMRC and other persons appointed by the Health Minister
- determines
the Strategy every five years and
- determines
the Priorities every two years, which the Health Minister must take into
account when making ‘decisions in relation to financial assistance’ granted
from the MRFF Special Account.[107]
Division 2 — Establishment of the
Advisory Board
Clause 32B establishes the Advisory Board. Clause
32C provides that the functions of the Advisory Board are:
- to
determine the Strategy and Priorities in accordance with Division 3 of
Part 2A of the Bill and
- advise
the Health Minister about other matters referred to the Advisory Board by the
Health Minister.
Subclause 32B(2) provides that the Health Minister
may give the Advisory Board written directions regarding the way in which it is
to carry out its functions, and the procedures to be followed at any meetings. Subclause
32B(3) provides that such a direction is not a legislative instrument.[108]
Clause 32D deals with the Strategy. Subclause
32D(1) provides that the Advisory Board must determine a strategy, the
purpose of which is to ensure ‘that a coherent and consistent approach is
adopted in providing financial assistance under this Act for medical research
and medical innovation’.
Factors to be considered in
developing the Strategy
Subclause 32D(3) provides that when determining the
Strategy, the Advisory Board must take into account the national strategy for
medical research and public health prepared by the NHMRC (NHMRC Strategy) and
any other relevant matters.[109]
Whilst it is not referred to in the Bill (as amended) itself, the Government
has indicated that when determining the Strategy, the Advisory Board should ‘also
refer to the government's science and research priorities’, suggesting that
this is an example of another ‘relevant matter’ that must be taken into
account.[110]
The NHMRC Strategy forms part of the NMHRC’s corporate plan.[111]
Relevantly, the corporate plan must set out an assessment by the CEO of the
NMHRC of the ‘major national health issues that are likely to arise’ during the
period covered, and also the NHMRC Strategy.[112]
As part of preparing the corporate plan (and therefore the NHMRC Strategy), the
CEO of the NMHRC must ‘consult with the Minister and the Council on the matters
proposed for inclusion in the plan'.[113]
How the Fund and NHMRC would
coordinate efforts
The Medical Research Endowment Account (MREA) is
administered by the NHMRC.[114]
The MREA is a special account established under the NHMRC Act.[115]
Payments are made from the MREA to government departments, universities,
institutions or people engaged in medical research and for assistance in
training people in medical research.[116]The
above arrangement in the Bill appears to be aimed at ensuring that the Strategy
does not result in replicating the priorities and programs funded by the MREA through
the NHMRC. Instead, it aims to ensure that the Strategy, Priorities and funding
provided through the Fund and that provided through the MREA complement each
other, with the Government stating:
The Australian medical research and innovation strategy will
ensure that a coherent and consistent approach is adopted in the funding of
medical research and medical innovation from the MRFF over a five-year period.
The strategy determined by the advisory board must take into
account the national strategy prepared by the NHMRC, and it should also refer
to the government's science and research priorities. The priorities will inform
the proposals for disbursement that I will take to cabinet each year for
decision through the budget process... The MRFF represents a major injection of
new funds into the medical research sector, adding to the research funding
allocated by the NHMRC through the Medical Research Endowment Account. The
MRFF and MREA will operate alongside each other and in clear sight of each
other. My biennial reports to the parliament will disclose how the spending
profile for the MRFF adds to other categories of Commonwealth funding on
medical research and innovation, to demonstrate that the new fund builds upon
existing funding.[117]
The importance of ensuring that the Fund and NMHRC
coordinate efforts and that the funding provided by the Fund complements,
rather than duplicates, that provided through the MREA was highlighted in a
number of submissions. The University of Tasmania (UTAS) noted:
The interaction between the NHMRC and the MRFF might require
greater consideration of how to generate the strategic research coordination to
influence the cultural, institutional and financial drivers of medical and
health research and their translation into clinical practice. In the UK this
approach occurs through the Office for Strategic Coordination of Health
Research that sits above their Medical Research Council and National Institute
for Health Research. This model might be considered in coordinating the MRFF.[118]
In summary, subclause 32D(3) provides for indirect Ministerial
input into the development of the Strategy, via the requirement to take into
account the NMHRC Strategy. However, this approach is not unusual (as
demonstrated by subsection 16(1) of the NMHRC Act) and the
Bill contains additional safeguards aimed at ensuring the Strategy is developed
at a reasonable arm’s length from the Government of the day. It would also
appear to at least partially address the concerns raised about possible
duplication of efforts and funding through the MREA and the Fund by ensuring that
in developing the Strategy and Priorities, the Advisory Board must take into
account the NHMRC Strategy.
Limitations on the content of the
Strategy
Subclause 32D(5) provides that the Strategy must not
‘require financial assistance to be provided to a particular person, or for a
particular project’. The Government states that this limitation will ensure
that:
The Strategy will not identify particular projects to receive
funding; in part this requirement ensures that the members of the Board who are
also members of the medical research community are not exposed to potential
conflicts of interest.[119]
In other words, subclause 32D(5) ensures that it
would be difficult for members of the Advisory Board to use the Strategy to
benefit a particular institute or entity they work for or are affiliated with. However,
it would appear that there may be circumstances in which the Strategy may have
the effect of ensuring that financial assistance is provided to a
particular person or for a particular project. For example if the NMHRC
Strategy identifies a particular emerging public health issue that was only being
researched by one team or organisation, then it is conceivable that the
Strategy may suggest funding it, thus effectively requiring (or at least
strongly recommending) that financial assistance be provided to a particular
entity or project (as there is only one relevant entity or project in existence).
Contents of the Strategy are fixed
for five years and cannot be amended
Subclause 32D(4) provides that the Strategy, once
developed, remains in force for five years. Further, subclause 32D(8)
provides that the Strategy cannot be repealed, rescinded, revoked or amended.
In other words, once developed, it remains in force and unalterable for
five years.[120]
In contrast, section 19 of the NMHRC Act provides
that the NMHRC corporate plan (and therefore the NMHRC Strategy) can be varied,
with approval of the Minister. The Government argues that preventing the
Strategy from being altered is justified on the basis that this approach will ‘support
a consistent and planned approach to medical research funding’.[121]
Subclause 32D(6) provides that whilst the Strategy
is a legislative instrument, it is not subject to disallowance. The Government
argues that ‘this approach enables the public and the Parliament to hold the
Advisory Board and the Government accountable without impeding the Advisory
Board’s ability to perform its functions’.[122]
Note that subclause 32D(7) also provides that the Strategy must be
published on the Internet.
Clause 32E deals with the Priorities, which must
be taken into account by the Health Minister when making funding decisions.[123]
The provision sets out the relationship of the Priorities with the Strategy and
how they are to be developed by the Advisory Board.
Factors to be considered in
developing the Priorities
Subclause 32E(1) provides that the Advisory Board
must determine the Priorities for providing financial assistance for medical
research and medical innovation. Subclause 32E(2) then provides that the
Priorities must be consistent with the Strategy. In addition to being
consistent with the Strategy, subclause 32E(3) provides that, in
determining the Priorities, the Advisory Board must take into account:
- the
burden of disease on the Australian community
- how
to deliver practical benefits from medical research and medical innovation to
as many Australians as possible
- how
to ensure that the financial assistance provided delivers the greatest value
for all Australians
- how
to ensure that the financial assistance provided complements and enhances other
financial assistance provided for medical research and medical innovation and
- any
other relevant matter.
Whilst it is not referred to in the Bill (as amended)
itself, the Government has indicated that when determining the Strategy, the
Advisory Board should ‘also refer to the government's science and research
priorities’.[124]
As the Priorities must be consistent with the Strategy, this suggests that the
science and research priorities of the government of the day (which are
different to the Priorities in the Bill) are an example of a ‘relevant matter’
that may be taken into account by the Advisory Board when developing the
Priorities.[125]
Limitations on the content of the
Priorities
In contrast to the limitation placed on the Strategy, clause
32E does not contain a specific prohibition on the Priorities requiring
financial assistance to be provided to a particular person, or for a particular
project. It is not clear why this approach has been taken, and no explanation
is given in the Supplementary Explanatory Memorandum. This would appear to
allow situations where the Advisory Board sets Priorities that may result in
financial assistance being provided to projects in which members of the
Advisory Board are involved (as some may be members of the medical research
community), and hence a conflict of interest arises. This issue is partially
resolved by clause 32K, as discussed below.
Contents of the Priorities are
fixed for two years
Subclause 32E(5) provides that the Priorities, once
developed, remain in force for two years. Further, subclause 32E(8)
provides that the Priorities cannot be repealed, rescinded, revoked or amended.
In other words, once developed, they remain in force and unalterable for
two years.[126]
The Government argues that preventing the Priorities from
being altered is justified on the basis that this approach will ‘support a
consistent and planned approach to medical research funding’.[127]
Subclause 32E(6) provides that whilst the Priorities
are a legislative instrument, they are not subject to disallowance. The
Government argues that ‘this approach enables the public and the Parliament to
hold the Advisory Board and the Government accountable without impeding the
Advisory Board’s ability to perform its functions’.[128]
Note that subclause 32E(7) provides that the Strategy must be
published on the Internet.
Issues arising from the five-year
and two-year fixed periods that the Strategy and Priorities remain in force
A number of submissions to the Senate inquiry noted an
inconsistency arising from the five-year and two-year fixed periods that the
Strategy and Priorities must remain in force. For example, the MRFFAG noted:
Given the five and two year timeframes, the Action Group
queries how the Priorities can remain consistent at all times with the Strategy
when there isn’t always the opportunity to renew the Priorities in the same
year the Strategy is renewed.[129]
Likewise, Professor Alan Pettigrew (the Inaugural CEO of
the NHMRC), making a submission in a private capacity, also noted that ‘it is
possible that the variation of priorities every two years would not enhance
stability in Australia’s approach to health and medical research’ and therefore
recommended that consideration be given to reviewing the Priorities every five
years, at the same time as the review of Strategy is undertaken.[130]
These are legitimate concerns, given that subclause 32E(2)
provides that the Priorities must be consistent with the Strategy. Put simply,
if a new Strategy is released whilst existing Priorities are in force and those
Priorities are inconsistent with the Strategy, subclause 32E(8) will
prevent them from being amended to be consistent with the Strategy, thus
creating a situation where the Priorities and Strategy are not aligned and
cannot be brought into alignment for a period of time.
Ensuring the Strategy and
Priorities are developed using evidence and are not politicised
A number of submissions made reference to how the Strategy
and Priorities are to be developed.[131]
UTAS expressed concern at the arrangements proposed by the Bill:
The proposed Bill should deliver a rigorous and transparent
mechanism for identifying national health and medical research priorities as
well as a strategy for their delivery (and reporting) through a competitive
funding process. Once identified the process for attracting and evaluating
research proposals would require a more explicit commitment to a national and
international peer-review system of quality control.
The Bill indicates the powerful role of an Advisory Board in
managing the MRFF and providing advice to government (through the Minister to
the Cabinet) about its use, but the membership, constituency and powers of this
Board are insufficiently clear. Additional effort is needed to ensure that
priority setting is evidence-based and driven by demonstrated need rather than
short-term political expediency.
Australian medical research is highly regarded
internationally and has received many accolades both to individuals and as a
system. We have also seen significant translation of publicly-funded basic
research into commercially successful products (e.g. Cochlear, ResMed,
Gardasil, CSL). Each of these areas of success has depended ultimately on the
nature of the decision-making for funding, which for the past 60 years has been
peer-review driven. We support continuation of such a system into the MRFF.[132]
In addition, other submissions suggested that the process
of developing the Strategy and Priorities, as well as being open and
transparent, include a process of consultation and input from consumers.[133]
Division 4 – the Advisory Board
Membership of the Advisory Board
Clause 32F provides that the Advisory Board consists
of the CEO of the NMHRC and up to seven ‘other’ members. The Minister must
appoint one of the members of the Advisory Board (other than the CEO of the
NMHRC) to be the Chair.[134]
Appointment of members
Clause 32G deals with the appointment of members to
the Advisory Board. A person is not eligible for appointment to the Advisory
Board unless the Health Minister is satisfied that their appointment ensures
that the Advisory Board collectively possesses ‘an appropriate balance of
experience and knowledge’ in the following fields:
- medical
research
- policy
relating to health systems
- management
of health services
- medical
innovation
- financing
and investment and
- commercialisation
of research and innovation.[135]
Members are appointed by the Health Minister by written
instrument, and serve on a part-time basis for a period of time specified in
the written instrument (not exceeding five years).[136]
They can, however, be reappointed after their term expires.[137]
The expertise of Advisory Board
members and nomination
A number of submissions expressed the view that it was
necessary for the Advisory Board to include a wider range of expertise than
that proposed in the Bill including: clinical trials, nursing, midwifery,
allied health, related scientific disciplines (such as chemistry),
hospital-based experts, clinical research (including health services research).[138]
In addition, the University of Western Sydney expressed a
desire for greater clarity around how Advisory Board Members are selected,
noting that the process for nominating members of the Advisory Board, and the
length and nature of their term, is not clear.[139]
Likewise the Australian Society for Medical Research (ASMR) stated:
... more information is required pertaining to the appointment
of board members and the relevant selection criteria. Such processes are
already in place via NHMRC and should be utilised.[140]
The need for consumer
representation on the Advisory Board
A number of submissions recommend that the Advisory Board
should include one or more consumer, patient advocacy, carer, or community
representative members.[141]
For example, the University of Notre Dame Australia recommended that ‘a
community/consumer perspective and voice on the Advisory Board is desirable’
and that the Board ‘should be able to access relevant independent expert advice
if required to support their considerations’.[142]
Similarly Cancer Voices Australia recommended that:
There is a lot of evidence that better decisions are made
when consumers are involved and the obvious benefit is community involvement in
allocating community and donor funds. Most large research funders in Australia
have recognised this and include consumers in their panels of people who
allocate research eg the various Cancer Councils, Cancer Institute NSW,
National Breast Cancer Foundation etc. It is also accepted that two, rather
than one, consumer voices should be the standard, best practice approach. We
were disappointed such an approach was dropped by the Government with the new
appointments to the Pharmaceutical Benefits Scheme Committee.
We strongly request that the Bill be amended to include two
consumer representatives on the Advisory Board, including one representing
Australians with cancer due to the size of its burden in terms of people
affected and costs.[143]
Remuneration
Clause 32H deals with the remuneration and allowances
of the members of the Advisory Board. As clause 32F provides that the
Advisory Board consists of the CEO of the NMHRC and ‘up to seven other
members’ (emphasis added) it can be inferred that a reference to a member of
the Advisory Board includes (unless specified otherwise) the CEO of the NMHRC.[144]
Subclause 32H(1) provides that ‘a member’ of the
Advisory Board (thus including the CEO of the NMHRC) is to be paid remuneration
determined by the Remuneration Tribunal, or, if no such determination has been
made, as determined by the Health Minister.[145]
In relation to allowances however, subclause 32H(2) provides that a
member of the Advisory Board is to be paid ‘allowances that are prescribed’
under subclause 32H(4), which allows the Health Minister to determine
such allowances by legislative instrument. This is somewhat unusual as
allowances for members of such bodies are usually determined by the
Remuneration Tribunal.[146]
Conflict of Interests
Clause 32K deals with the disclosure on interests by
Advisory Board members. It applies to a member who has a ‘material personal interest’
in a matter being considered (or about to be considered) by the Advisory Board.[147]
As is standard with similar legislative provisions:
- a
member must, as soon as possible, disclose the nature of the material personal
interest at a meeting of the Advisory Board and also to the Health Minister[148]
- the
disclosure must be recorded in the minutes of the relevant meeting[149]
and
- where
a member, without reasonable excuse, fails to disclose a material personal
interest, the Health Minister must terminate their appointment.[150]
However, unlike analogous provisions in legislation dealing
with the disclosure and management of material personal interests by members of
boards or committees, clause 32K does not prohibit a member of
the Advisory Board who has disclosed a material personal interest from being
present when the matter is considered or from taking part in any decision made
in relation to the matter. (Such matters could be addressed in the directions
that may be given to the Board by the Health Minister under subclause 32B(2).)
In contrast, this issue is directly addressed in other legislation, as
demonstrated by the table below.
Table 2: disclosure of conflict of
interest and voting
|
Act, Regulation or Bill
|
Relevant provision
|
Duty imposed in relation to voting
|
|
The Bill
|
Clause 32K
|
No restrictions on voting on a matter pertaining to a
disclosed material personal interest.
|
|
Public
Governance, Performance and Accountability Rule 2014 (made under the Public
Governance, Performance and Accountability Act 2013 (PGPA Act 2013))
|
Rule 15 and Section 29 of the PGPA Act 2013
|
An official who has a material personal interest in a
matter that relates to the affairs of the entity must not (subject to certain
exceptions):
- be
present while the matter is being considered at the meeting or
- vote
on the matter.
|
|
Future
Fund Act 2006
|
Section 71
|
A Board member who has a material personal interest in a
matter that is being considered at a Board meeting must not (subject to
certain exceptions):
- be
present while the matter is being considered at the meeting or
- vote
on the matter.
|
|
NMHRC Act
|
Subsection 42A(5)
|
A member who has disclosed an interest in a matter under consideration
must not (unless the CEO or Chair otherwise determines):
- be
present when the Council or committee considers the matter or
- take
part in any decision of the Council or committee in relation to the matter.
|
|
Corporations
Act 2001
|
Section 195
|
A director of a public company who has a material
personal interest in a matter that is being considered at a directors'
meeting must not (subject to certain exceptions):
- be
present while the matter is being considered at the meeting or
- vote
on the matter.
|
|
Fair Work (Registered Organisations) Amendment Bill 2014
[No. 2]
|
Proposed section 293F
|
An officer of an organisation who has a material personal
interest in a matter that relates to the affairs of the organisation:
- must
not be present during any deliberation by the organisation on the matter and
- must
not take part in any decision of the organisation with respect to the matter.
|
No rationale appears to have been provided for why the
legislation does not specify that members of the Advisory Board are not allowed
to vote on matters in which they have a material personal interest, or why the
approach adopted by clause 32K differs from the ‘standard’ approach
observed in other Commonwealth legislation, such as the PGPA Act 2013, Corporations
Act 2001, Future Fund Act 2006, NMHRC Act and that proposed by
the Fair Work (Registered Organisations) Bill 2014 [No. 2]. (Although, as set
out above, this could be dealt with in directions on the procedures to be
followed in Advisory Board meetings, given by the Health Minister under subclause
32B(2), it would be clearer and ensure greater transparency if the
requirements were included in the legislation itself.)
Termination of Advisory Board
members
Clause 32N provides that the Health Minister may
terminate the appointment of a member of the Advisory Board (other than the CEO
of the NHMRC). Unlike analogous provisions in legislation dealing with the
termination of board members, clause 32N does not restrict termination
to a pre-defined set of circumstances such as bankruptcy, misbehaviour or
mental or physical incapacity, as demonstrated by the table below.
|
Act
|
Relevant provision
|
Grounds for termination
|
|
The Bill
|
Clause 32N
|
None specified. On the face of the Bill, the Health
Minister has an unfettered power to terminate the appointment of any member
for any reason, at any time. (Subject to standard administrative law considerations.)
|
|
Future Fund Act 2006
|
Section 44
|
A Board member can only be terminated for:
- misbehaviour
- physical
or mental incapacity
- becoming
bankrupt or
- certain
other circumstances.
|
|
NMHRC Act
|
Section 44B
|
A Board member can only be terminated for:
- misbehaviour
- physical
or mental incapacity
- becoming
bankrupt or
- certain
other circumstances.
|
No rationale appears to have been provided for why the
Health Minister requires such a wide power to terminate the appointment of Advisory
Board members, nor why the approach adopted by clause 32N differs from
the approach observed in the Future Fund Act 2006 and NMHRC Act.
Part 3 – Maximum annual distributions
Part 3 of the Bill deals with the maximum annual
distribution (MAD) that can be debited from the MRFF Special Account each
financial year.[151]
Subclause 34(1) provides that the FFB must determine the MAD for each
financial year by the day specified by the Finance Minister.[152]
The determination produced by the FFB must also include the methods used for
working out the MAD and the considerations taken into account when working out
that amount, thus providing a degree of transparency if the Finance Minister
chooses to publish the determination on the Internet (see below).[153]
When determining the MAD, subclause 34(4) provides that the FFB must
take into account a number of factors including:
- preserving
the long-term nominal value of financial assets initially transferred from the
HHF to the Fund[154]
- preserving
the long-term nominal value of the total amount of other amounts credited to
the Fund[155]
- moderating
(to the extent possible) year-on-year variability of the MAD[156]
- maintenance
of the FFB’s ability to comply with the Fund Investment Mandate (Investment
Mandate) issued under clause 39[157]
- any
costs, expenses, obligations or liabilities likely to be incurred by the FFB
for the purposes of clauses 18 and 19 (expenses related to the Investment
Portfolio)[158]
and
- any
additional matters specified by the Finance Minister.[159]
The Explanatory Memorandum notes that ‘the principle of
preserving the nominal value of capital invested in the Medical Research Future
Fund by the Government is to be applied over a long-term time horizon’.[160]
The Bill also provides that the Finance Minister has the discretion to publish
the FFB’s determination on the Internet, and clarifies that the determination
is not a legislative instrument (and hence is not subject to disallowance).[161]
Part 4 – Investments of the Fund
Part 4 of the Bill contains the bulk of the
provisions that deal with the proposed governance structure of the Fund’s
Investment Portfolio, what it can invest in (and for what purposes), the role
of the FFB and the degree of oversight and direction that can be provided by
the responsible Ministers.
Where does the Fund get the funds
to invest?
Subclause 37(1) provides that the FFB uses amounts
standing to the credit of the MRFF Special Account to fund its investments. Clause
38 then provides that income, return of capital, and the proceeds of realised
investments must be credited to the MRFF Special Account. In short, income and
returns from the Fund’s investments provide the funding for making investments.
What can the Fund invest in?
Investments made from the MRFF Special Account are made by
the FFB on behalf of the Fund.[162]
Those investments must be made in the name of the FFB, but are taken to be
investments of the Fund.[163]
Subclause 37(1) provides that the FFB can invest amounts standing to
the credit of the MRFF Special Account in any financial assets – a term
defined expansively in section 6 of the Future Fund Act 2006 - which in
turn can be expanded by regulations issued under that Act.[164]
As result, the Fund can, for example, invest in:
- cash
and deposits (both Australian currency and foreign currency)
- investments,
loans and placements (include bonds, debentures, various forms of loans and
redeemable preference shares) and
- equity
(shares in listed companies, preference shares and other claims on other
entities entitling the holder to a share of the income of the entity and a
right to a share of the residual assets of the entity should it be wound up).[165]
Clause 47 also provides that the FFB, for certain
purposes (such as protecting the value of an investment) can invest in derivatives.[166]
Clause 49 also allows the FFB to enter into securities lending
arrangements for a purpose connected with the Fund.
How does the Fund purchase
investments?
Clause 50 allows the FFB to engage one or more
investment managers for purposes in connection with the Fund. The Explanatory
Memorandum notes that ‘an investment manager is defined broadly to include
custodians, transition managers and other investment managers.’[167]
However, it also notes that the Future Fund Management Agency (Agency) is
excluded from the definition of an Investment Manger as ‘it is generally
expected that investment activities, such as acquiring derivatives or investing
money, will be outsourced.’[168]
Subclause 50(2) then provides that the FFB only:
- invest
amounts under subclause 37(1) (discussed above)
- acquire
derivatives under subclause 47(1)
- enter
into securities lending arrangements under subclause 49(1) or
- realise
financial assets that are investments of the Fund
through an investment manager engaged by the FFB or in a
manner approved by the responsible Ministers.
The Bill also provides that the FFB must ensure that any
investment managers it engages operate consistently with the Bill, and provide
reports regularly to it and the Agency.[169]
This approach to making investments is consistent with that adopted in the Future
Fund Act 2006.
As noted in the preamble to the Bill, the purpose of establishing
the Fund is to generate ‘income over the long term’ as that is ‘the most
appropriate mechanism’ for ensuring that funding is available for medical
research and innovation.[170]
This view is reinforced by clause 40, which states that in performing
its Investment Portfolio function, the FFB has an obligation to seek to:
- maximise
the return earned on the Investment Portfolio over the long term (consistent
with international best practice for institutional investment) and
- enhance
the Commonwealth’s ability to provide grants of financial assistance to support
medical research and medical innovation.
As a result, in simple terms, the object of the Investment
Portfolio is to generate income and returns which are then credited to the MRFF
Special Account to provide the funding for:
- further
investments in additional or other financial assets
- the
provision of grants and payments for medical research and medical innovation
under Part 2, Division 4, subdivisions B, C, D and E (discussed
above) and
- enhancing
the ability of FFB to discharge costs, expenses, obligations and liabilities
and make payments for the additional purposes set out in clauses 18 and
19 (discussed above).[171]
However, when performing its Investment Portfolio function
and pursuing those objectives, the FFB remains subject to the Bill as a whole
and the Investment Mandate (discussed below) which prevails over the obligation
imposed by clause 40, to the extent of any inconsistency.[172]
The Investment Mandate, governance
of the Fund and Ministerial oversight
The Bill creates a two-tiered governance structure. The
Government, through the responsible Ministers, provides broad ‘strategic
guidance’ to the FFB on how it expects the Fund to be invested. In turn, the FFB
is responsible for developing policies to guide its investment decisions, which
are then carried out by Investment Managers engaged for that purpose.
Importantly however, the responsible Ministers are not able to direct the FFB
to invest in a particular financial asset, business entity or activity.[173]
Whilst this approach is consistent with that adopted under the Future Fund
Act 2006, it differs from the grant approval process (discussed above) where
the relevant Minister can choose to direct funds through a grant or payment to
specific entities of their choosing.[174]
Clause 39 deals with the Investment Mandate, in
similar terms to section 18 of the Future Fund Act 2006. In effect, the
Investment Mandate ‘provides the Government, as owner of the Medical Research
Future Fund, with a mechanism for articulating its expectations for how the
Medical Research Future Fund will be invested and managed by the Future Fund
Board’.[175]
The Government notes that the framework established by clause 39 that
enables it ‘to give strategic guidance’ to the FFB whilst preserving the FFB’s
‘role in managing’ the Investment Portfolio ‘at arm’s length from the
Government’ is ‘consistent with the arrangements in place for the Future Fund’.[176]
The Investment Mandate is a written direction to the FFB
produced by the responsible Ministers about the performance of its Fund
investment functions.[177]
Subclause 39(1) ensures that the responsible Ministers (the Minister for
Finance and the Treasurer) must produce at least one Investment Mandate. In
producing the Investment Mandate, the responsible Ministers ‘must have regard
to’:
- maximise
the return earned on the Fund over the long term, consistent with international
best practice for institutional investment
- enhance
the Commonwealth’s ability to provide grants of financial assistance to support
medical research and medical innovation and
- any
other matters the responsible Ministers consider relevant.[178]
However, clause 42 provides that the Ministers must
give the FFB a draft of the Investment Mandate, invite the FFB to make
submissions on it (within a timeframe set by the responsible Ministers) and
consider submissions made within that time limit. The submissions must be
tabled in each House of Parliament, along with the final Investment Mandate.
Whilst the Investment Mandate is a legislative instrument, it is not subject to
disallowance or sunsetting.[179]
Overall, clause 42 provides a degree of transparency as the requirement
to table the FFB’s submissions along with the Investment Mandate will highlight
any disagreements between the approach recommended by the FFB and that desired
by the Government. However, the Bill does not provide for any formal parliamentary
input into the process of developing the Investment Mandate, reflecting the
position under the Future Fund Act 2006.[180]
Whilst subclause 43(1) provides that the FFB must take ‘all reasonable
steps’ to comply with the Investment Mandate, subclause 43(5) provides
that a failure to do so will not affect the validity of any transaction.
Subclause 39(4) provides that the Investment Mandate
can set out the policies to be pursued by the FFB in relation to matters of
risk and return and the allocation of financial assets, and any policy relating
to the allocation of financial assets ‘must not be inconsistent with a policy
relating to matters of risk and return’. Subclause 39(5) provides that
the Investment Mandate prevails over the obligations imposed by clause 40
(to the extent of any inconsistency) and must not otherwise be inconsistent
with the Bill.
Clause 41 places limitations on the contents of the Investment
Mandate.[181]
Under subclause 41(1), the responsible Ministers must not give a
direction within the Investment Mandate which would, directly or indirectly,
require the FFB to invest in a particular financial asset, acquire a particular
derivative, or allocate finances to a particular business entity, activity or
business.[182]
As such, subclause 41(1) will prevent the
Investment Portfolio being used as a political ‘slush fund’. A further
transparency measure is included in clause 46. Briefly, clause 46
imposes an obligation on the FFB to produce and comply with written policies that
are consistent with the Investment Mandate in relation to:
- the
investment strategy for the Investment Portfolio
- the
benchmarks that are used to assess the performance of the Investment Portfolio
- the
risk management strategies of the Investment Portfolio and
- matters
relating to international best practice for institutional investment or
specified in the rules.[183]
Subclauses 46(3) and (4) provide that
such policies must be published in the internet, thus giving a degree of
transparency around how the FFB ‘performs its investment function’ in relation
to the Investment Portfolio ‘including risk management, performance assessment
and benchmarks’.[184]
The need for the Investment Mandate
to focus on or prohibit certain types of investments
A number of submissions suggested that the Bill be amended
to focus the investment activities undertaken by the Future Fund Board (FFB) in
relation to the Investment Portfolio to either encourage investment in certain
types of industries, or prohibit investment in industries that have negative
health impacts.[185]
For example, Orygen — The National Centre of Excellence in Youth Mental Health,
recommended that:
... in describing the investment strategy used to generate
returns to the MRFF:
- there could be scope to invest in commercialising/translation companies
-
there could be a restriction on investing in organisations producing products
that are damaging to health (e.g. alcohol)[186]
The Medical Research Future Fund Action Group (MRFFAG)
also suggested that clause 36 of the Bill be amended to provide another
ancillary object of supporting ‘medical innovation’.[187]
The MRFFAG argued that such an amendment ‘together with clause 39(2)(c) would
then allow the responsible Ministers to provide the Future Fund Board with a
direction in the Investment Mandate regarding investment in medical
innovation.’[188]
Part 5 – Reporting obligations
Part 5 deals with reporting obligations and other
transparency measures that are consistent with those found in the Future
Fund Act 2006.[189]
Clause 55 allows the Finance Minister to require the FFB to prepare a
report or document about the performance of the FFB’s functions within a
specified timeframe, and discretion as to whether to publish such reports or
documents.
Clause 56 imposes an obligation on the FFB to keep
the responsible Ministers informed about its operations, and to provide
reports, documents or information in relation to those operations as
appropriate. Clause 57 then allows the Finance Minister to give a
Minister any such reports, documents or information (as well as other information
or documents obtained by the Finance Minister under the Bill). Clause 57A
requires the Health Minister to prepare a report on the financial assistance
provided from the MRFF Special Account against the Priorities every two years.
The report must be tabled in both Houses of Parliament and include:
- a
description of how the financial assistance provided was consistent with the
Priorities and
- information
about any other financial assistance provided by the Commonwealth for medical
research and medical innovation.[190]
The Victorian Government recommended that the Bill be
amended to provide that the Health Minister must report to parliament on the
financial assistance provided from the MRFF Special Account on an annual,
instead of biennial basis.[191]
Whilst the Government has stated that a report prepared
under clause 57A must ‘include a description of how the financial
assistance provided was consistent with the Strategy and Priorities’, the text
of the Bill itself does not require that the report outline the consistency of
financial assistance provided with the Strategy, only the Priorities.[192]
As such, from a strictly legal perspective a report prepared under clause
57A need not include a description of how financial assistance provided was
consistent with the Strategy.
Clause 58 provides that the Health Minister must
publish information on the internet about grants provided from the Health
Account to entities of the kind referred to in subclause 26(1) — that
is, medical research institutes, universities, corporate Commonwealth entities
and corporations.
Part 6 – Miscellaneous
Delegation
Clauses 60, 61 and 61A allow the Finance Minister,
Health Minister and Treasurer to delegate certain powers under the Bill to high
ranking officials. For example, this includes the Health Minister’s powers to
issue directions to facilitate the making of grants or payments to states,
territories, corporate Commonwealth entities and specific entities of certain
types (such as medical research institutes).[193]
Certain aspects of these powers can be delegated to persons who must then
comply with any Ministerial directions.[194]
In effect, the Bill as amended by the Government allows the relevant Minister
to not only make a direction to issue a grant or make a payment to a specific
state, territory, entity or organisation, but to also delegate that power.
Delegation by the Health Minister
Clause 61A provides that Health Minister may, by
writing, delegate any or all of his or her powers under:
- clause
15A: requiring the Finance Minister to debit the MRFF Special Account
- clause
26: debiting the Health Account for the purposes of making the grant to an
entity of the type listed (a medical research institute, university,
corporation or corporate Commonwealth entity) and
- clause
27: drafting the terms and conditions of such grants and entering into the
agreement on behalf of the Commonwealth.[195]
Clause 61A allows the Health Minister to delegate
any or all of these powers to the Secretary of the Department of Health, the
CEO of the NHMRC or to Senior Executive officers in either organisation, with subclause
61A(2) indicating that anyone to whom these powers are delegated must
comply with Ministerial directions.
Delegation by the Finance Minister
Clause 60 provides that Finance Minister may, by
writing, delegate any or all of his or her powers under:
- clause
15: determining an amount that is to be credited to the MRFF Special
Account
- clause
20: directing that a specified amount be debited from the MRFF Special
Account and credited to the COAG Reform Fund for the purpose of providing a
specific grant to a state or territory for medical research and medical
innovation
- clause
25: directing that a specified amount be debited from the MRFF Special
Account and credited to the Health Account for the purpose of providing a grant
to an entity of the kind referred to in clause 24 and
- clause
29: directing that a specified amount be debited from the MRFF Special
Account for the purpose of providing a grant to a corporate Commonwealth entity
for the ‘purposes of supporting’ medical research and medical innovation.
Delegation by the Treasurer
Clause 61 provides that Treasurer may, by writing,
delegate any or all of his or her powers under:
- clause
15: determining an amount that is to be credited to the MRFF Special
Account and
- subclause
21(1): debiting the COAG Reform Fund for the purpose of providing a
specific grant to a state or territory for medical research and medical
innovation.
Embedded review
Clause 62 provides that the Bill will be subject to a
legislatively embedded review (LER). Specifically, the Minister must cause a
review of the operation of the Bill to be undertaken either:
- before
30 June 2023 or
- at
another date that the responsible Ministers consider appropriate.
It does not provide any details about who is to conduct the
review, what is to be examined, if the report of such a review is to be tabled
in Parliament and so forth. As a result, the timing and scope of any such
review is effectively at the discretion of the responsible Ministers. This is
not surprising, given that the Productivity Commission noted in 2011 that ‘there
do not appear to be any rules or guidelines about when an embedded review
should be included in Australian Government legislation, nor about the scope of
any such review’.[196]
It also noted that:
The scope of statutory reviews can vary substantially. The
terms of reference for the review may be set out in legislation or open to the
agency required to commission the review ... [t]he need for an embedded statutory
review is identified during the development of the regulation. As far as the
Commission is aware, this is done on an ad hoc basis by the departments
drafting the legislation ...[197]
As a result, whilst the LER provision provided by clause
62 is lacking in substantive details, it cannot be said to be a departure
from the usual form of such provisions – as none exists. However, the power
provided to the Minister to postpone the date by which the review is to be
conducted (potentially indefinitely) provided by paragraph 62(b) appears
to be highly unusual.[198]
Rule-making power
Clause 63 allows the Finance Minister, via a
legislative instrument, to make rules required, permitted, necessary or
convenient for carrying out and giving effect to the Bill.
Subclause 45(1) prohibits the FFB from borrowing
money except in the circumstances provided for in subclauses 45(2)
and (3) (for example, short term borrowing associated with the settlement
of transactions).[199]
The Explanatory Memorandum notes that ‘the overall aim of this section is to
ensure that the Future Fund Board is able to operate efficiently without
exposing the budget to undue risk’.[200]
Clause 51 deals with the tax treatment of franking
credits arising from investments in the Investment Portfolio. Its operation is
adequately described on page 25 of the Explanatory Memorandum.
Clause 52 provides that the FFB must realise an asset
that ceases to be a financial asset or any asset acquired by it (as an
investment of the Fund) that is not a financial asset. The Explanatory
Memorandum notes that this could occur in circumstances where the FFB:
... holds an asset which was mistakenly acquired by the Board,
or given to the Board, or which ceases to be a financial asset due to a
revision of the Australian Bureau of Statistics Government Finance
Statistics Manual...[201]
Clause 53 provides that a function of the FFB includes
investing amounts in accordance with the Bill.
The Medical Research Future Fund (Consequential
Amendments) Bill 2015 (Consequential Bill) makes consequential amendments to
the COAG Reform Fund Act 2008, Future Fund Act 2006, Nation-building
Funds Act 2008, DisabilityCare
Australia Fund Act 2013 and the Health Insurance Act
1973 for the purpose of:
- enabling
grants to the states and territories through the COAG Reform Fund
- extending
the FFB’s duties to manage the Fund and
- allowing
amounts to be transferred between the Fund and the Future Fund to allow for
proper apportioning of common expenses incurred by the FFB in managing the Fund,
Future Fund, Nation-building Funds and the DisabilityCare Australia Fund (DCAF).[202]
In addition, the Consequential Bill abolishes the HHF and creates
a special appropriation that will allow the Department of Health to meet
ongoing financial commitments related to HHF projects already entered into before
the HHF is closed.[203]
Structure of the Consequential Bill
The Consequential Bill has three schedules. Schedule 1
contains the main amendments that support the establishment of the Fund. Schedule
2 contains the amendments related to the abolition of the HHF. Schedule
3 contains amendments that are contingent on the commencement of certain
parts of the Acts and Instruments (Framework Reform) Act 2015.
Schedule 1
Amendments to the COAG Reform
Fund Act 2008
Items 1 to 3 make consequential amendments
that:
- recognise
that grants from the Fund to the States and Territories are channelled through
the COAG Reform Fund[204]
- allow
an amount that originates from the Fund to be transferred to the COAG Reform
Fund[205]
and
- provide
that grants provided to states and territories through the COAG Reform Fund are
subject to the provisions of the Bill.[206]
Further information on these amendments can be found on
page 6 of the Consequential Bill’s Explanatory Memorandum.
Amendments to the DisabilityCare
Australian Fund Act 2013
Items 4 to 6 make consequential amendments
that provide that certain expenses incurred by the FFB (such as establishing
and operating bank accounts, insurance premiums and expenses incurred by the FFB
in relation to managing the DCAF) can be met from the DCAF Special Account
provided:
- they
relate to the DCAF and
- only
to the extent that such expenses do not relate to any other fund (including the
Fund).
Further information on these amendments can be found on
page 7 of the Consequential Bill’s Explanatory Memorandum.
Amendments to the Future Fund
Act 2006
Items 7, 13 and 14 confirm that the FFB
has additional functions under the Bill related to the Fund.
Investment managers
Items 11, 12 and 20 will allow
investment managers engaged with the management of the Fund to also be engaged
for the purposes of managing a range of other funds and for the FFB to delegate
its power to engage such investment managers (in relation to the Fund) to the
Chair of the FFB or Senior Executive employees of the Agency.
Further information on these amendments can be found on
pages 7 and 8 of the Consequential Bill’s Explanatory Memorandum.
Reporting obligations
Items 15 and 18–19 deal with reporting
obligations. Item 15 clarifies that the FFB’s reporting obligation in
relation to the Future Fund are separate from the reporting obligations under
the Bill. Items 18 and 19 require that the annual report prepared
by the Chair of the FFB must include a report on:
- the
performance of the Investment Portfolio[207]
- the
amounts debited from the MRFF Special Account for purposes allowed under the Bill[208]
and
- must
include benchmarks in relation to the performance of the debits from the MRFF
Special Account.[209]
Further information on these amendments can be found on
pages 7 and 8 of the Consequential Bill’s Explanatory Memorandum.
Inter-fund transfers
Items 21 to 22 amend section 84 of the Future
Fund Act 2006 to provide that where money is received by the FFB and no
provisions in the relevant legislation cited require it to be credited to a
specific fund’s special account (including the MRFF Special Account), that amount
must be credited to the Future Fund Special Account. Item 23 then
provides that the Minister can direct that such amounts that are credited to
the Future Fund Special Account are subsequently transferred to the MRFF
Special Account. (Equivalent provisions already exist for the other funds
managed by the FFB.) Under subsection 84(6), which is amended by item 25,
such a written direction ‘does not have the status of a legislative instrument’
(and hence is therefore not disallowable by either House of Parliament). The
Explanatory Memorandum notes that this approach is:
... consistent with the status of other written directions that
can be made by the nominated Minister under subsections 84(2), 84(3), 84(4) and
84(4A) [of the Future Fund Act 2006] in relation to the transfer of
amounts to other Special Accounts as provided under those subsections.[210]
Item 31 adds a new clause to the end of Schedule 2A
(which deals with inter-fund transfers) allowing the relevant Minister to
provide a written direction requiring the reversal of a debit from the MRFF Special
Account to meet bank account, insurance premium or other costs relating to the Fund.
Such a reversal:
- is
to be effected by debiting the Future Fund Special Account and crediting the
MRFF Special Account and
- cannot
exceed the amount originally debited from the MRFF Special Account.
Proposed subclause 6(3) then provides that such a written
direction is not a legislative instrument.
Further information on these amendments can be found on
page 8 of the Consequential Bill’s Explanatory Memorandum.
Amendments to the Nation-building
Funds Act 2008
Sections 20, 138 and 219 of the Nation-building Funds
Act 2008 set out the additional purposes of, respectively, the:
- Building
Australia Fund (BAF)
- Education
Investment Fund (EIF) and
- HHF.
The provisions specify that expenses incurred by the FFB (such
as establishing and operating bank accounts, insurance premiums etc.) can be met
from the BAF, EIF or HHF Special Account (as appropriate) provided they relate
to the BAF, EIF or HHF and only to the extent that such expenses do not relate
to any other fund. Items 32 to 40 make consequential amendments to
these provisions to include references to the MRRF.
Further information on these amendments can be found on
page 9 of the Consequential Bill’s Explanatory Memorandum.
Schedule 2 — Abolition of the HHF
Item 38 repeals Chapter 4 of the Nation-building
Funds Act 2008, thus abolishing the HHF. Item 39 is a transitional
provision that ensures that agreements relating to payments from the HHF in
force under section 261 of the Nation-building Funds Act 2008
immediately before the repeal of that section are taken to have been made under
proposed section 46AB of the Health Insurance Act 1973 (which
will be inserted by item 20 of Schedule 2 to the Consequential
Bill). This will ensure that the agreements can continue to operate until such
time as they expire.
Items 1 to 4 make consequential amendments to
the COAG Reform Fund Act 2008 reflecting the cessation of the HHF. Items
5 to 7 make consequential amendments to the DisabilityCare
Australia Fund Act 2013 reflecting the cessation of the HHF. Items 8 to
19 make consequential amendments to the Future Fund Act 2006
reflecting the cessation of the HHF. Item 20 inserts a proposed Part
IVAA and proposed sections 46AA, 46AB, 46AC and 46AD into the
Health Insurance Act 1973 in relation to payments relating to the
HHF, which will allow outstanding commitments of the HHF to be met. Items 24
to 37 make consequential amendments to the Nation-building Fund
Act 2008 reflecting the cessation of the HHF. Further information on these
amendments can be found on page 12 of the Consequential Bill’s Explanatory
Memorandum.
Further information on these amendments can be found on
pages 10 to 12 of the Consequential Bill’s Explanatory Memorandum.
Schedule 3
Item 1 amends Schedule 3 of the Bill to change the
references to the Legislative Instruments Act 2003 to the Legislation
Act 2003, reflecting the commencement of the Acts and Instruments
(Framework Reform) Act 2015.
It would appear likely that the Bill will be the subject
of considerable debate in the Senate. In particular, the definitions of
‘medical research’ and ‘medical innovation’, as well as the desirability of
funding decisions being made by the Health Minister and through Cabinet
processes instead of being made by independent experts via peer review are
likely to be issues of considerable interest and potential diversity of views.
The process of nominating Advisory Board Members, the
mixture of expertise, consumer representation and how the Strategy and
Priorities are to be developed would also appear to be issues of concern.
Further, the desirability of allowing Advisory Board members to vote on matters
in which they have a material personal interest may also attract some debate.
Members, Senators and Parliamentary staff can obtain
further information from the Parliamentary Library on (02) 6277 2500.
[1]. Medical
Research Future Fund Bill 2015, preamble.
[2]. Acts and Instruments
(Framework Reform) Act 2015, accessed 7 August 2015.
[3]. Australian
Government, ‘Medical
Research Future Fund’, Budget overview, 2014–15, accessed 11 June
2015; Australian Government, ‘Medical
Research Future Fund’, Overview: Health, 2014–15, accessed 11 June
2015.
[4]. Australian
Government, ‘Medical
Research Future Fund’, Budget Overview, 2014–15, op. cit.
[5]. Explanatory
Memorandum, Medical
Research Future Fund Bill 2015, p. 4, accessed 15 June 2015.
[6]. Parliamentary
Joint Committee on Human Rights (PJCHR), Twenty-third
Report of the 44th Parliament , 18 June 2015, pp. 1-2,
accessed 7 August 2015. See also: PJCHR, Index
of bills considered by the committee in 2015, 25 June 2015, p. 16,
accessed 7 August 2015.
[7]. Senate
Community Affairs Legislation Committee, Inquiry
into the Medical Research Future Fund Bill 2015 and the Medical Research Future
Fund (Consequential Amendments) Bill 2015, The Senate, Canberra, 2015,
accessed 8 August 2015.
[8]. Senate
Standing Committee for the Scrutiny of Bills, Alert
Digest No. 6 of 2015, The Senate, Canberra, 17 June 2015, pp. 34–35,
accessed 18 June 2015.
[9]. Senate
Selection of Bills Committee, Report
No. 6 of 2015, The Senate, Canberra, 16 June 2015, p. 4, accessed 18
June 2015.
[10]. Senate
Selection of Bills Committee, Report
No. 6 of 2015, op. cit., p. 7. See
also: D Bushby, ‘Selection of Bills Committee Report’, Senate, Debates,
16 June 2015, p. 3544 (moving that Report No. 6 of 2015 be
adopted); C Moore, ‘Selection of Bills Committee Report’, Senate,
Debates,
16 June 2015, p. 3546 (moving the amendment that the Bills be referred to the Community
Affairs Committee), Division
at 15.50 (33 Ayes, 27 Noes), amendment passed’, all accessed 7 August
2015.
[11]. C
King, ‘Consideration
in detail: Medical Research Future Fund Bill 2015’, House of
Representatives, Debates, 4 June 2015, p. 5886, accessed 16 June 2015, ‘We
want to support medical research. We want to support a Bill, but we cannot
support this Bill in its current form. It must be amended.’
[12]. C
King, ‘Second
reading speech: Medical Research Future Fund Bill 2015’, House of
Representatives, Debates, 4 June 2015, p. 5855, accessed 16 June 2015.
[13]. C
King (Shadow Minister for Health), Abbott
must ensure MRFF cannot be used as a $20 billion Coalition Fund,
media release, 4 June 2015, accessed 15 June 2015.
[14]. The
amendments moved by the Opposition can be accessed via the Medical Research
Future Fund Bill 2015 homepage here.
See also: C King, ‘Consideration
in detail: Medical Research Future Fund Bill 2015’, op. cit., p. 5886.
[15]. M
Butler (Shadow Minister for Environment, Climate Change and Water), ‘Consideration
in detail: Medical Research Future Fund Bill 2015’, House of
Representatives, Debates, 4 June 2015, p. 7030, accessed 21 July 2015.
[16]. Z
Wang, ‘Money
must be found for the medical research future fund’, The Australian,
2 March 2015, p. 10, accessed 15 June 2015.
[17]. A
Bandt (Greens Deputy Leader), Abbott
and Cormann need 'Plan B' on Medical Research Fund, media release,
[22 August 2014], accessed 16 June 2015.
[18]. Explanatory
Memorandum, Medical
Research Future Fund Bill 2015, op. cit., p. 4.
[19]. R
Lewis and J Kelly, ‘”Goodies”
and “baddies” in crossbench sights’, The Australian, 14 May 2015, p.
6, accessed 16 June 2015.
[20]. Senate
Community Affairs Legislation Committee, Inquiry into the Medical Research
Future Fund Bill 2015 and Medical Research Future Fund (Consequential
Amendments) Bill 2015, Submissions,
accessed 25 July 2015.
[21]. J
Hockey, ‘Second
reading speech: Medical Research Future Fund Bill 2015’, House of
Representatives, Debates, 27 May 2015, p. 4701, accessed 8 August
2015.
[22]. Explanatory
Memorandum, Medical
Research Future Fund Bill 2015, op. cit., p. 6.
[23]. R
Gittins, ‘Medical
research future fund: How the trick is done’, Sydney Morning Herald,
(online edition), 15 December 2014, accessed 16 June 2015.
[24]. The
Statements of Compatibility with Human Rights can be found at page 6 of the
Explanatory Memorandum to the Bill and page 5 of the Explanatory Memorandum to
the Consequential Bill.
[25]. See:
Medical Research Future Fund Bill 2015, Government amendments [sheet
HK145] and Medical Research Future Fund (Consequential Amendments) Bill
2015, Government amendments [sheet
ZA395], accessed 8 August 2015.
[26]. S
Ley, ’Consideration
in detail: Medical Research Future Fund Bill 2015’, House of
Representatives, Debates, 22 June 2015, p. 7029, accessed 13 July 2015.
[27]. The
inclusion of the type of provision is becoming increasingly common. See, for
example: section 333 of the Navigation Act 2012;
section 4 of the National
Land Transport Act 2014 and section 206 of the National Disability
Insurance Scheme Act 2013, all accessed 8 August 2015.
[28]. Pape v
Commissioner of Taxation [2009] HCA 23; (2009) 238 CLR 1; Williams v
Commonwealth of Australia [2012] HCA 23; (2012) 248 CLR 156 and Williams v
Commonwealth of Australia [2014] HCA 23; (2014) 252 CLR 416.
[29]. Williams v
Commonwealth of Australia [2012] HCA 23; (2012) 248 CLR 156, as per
Hayne J at [194].
[30]. Financial Framework
Legislation Amendment Act (No. 3) 2012, accessed 8 August 2015.
[31]. Explanatory
Memorandum, Medical
Research Future Fund Bill 2015, op. cit., p. 8.
[32]. Constitution,
accessed 8 August 2015.
[33]. The
University of Melbourne, Submission
to the Senate Community Affairs Legislation Committee, Inquiry into the Medical
Research Future Fund Bill 2015 and Medical Research Future Fund (Consequential
Amendments) Bill 2015, no date, pp. 2, 4; Innovative Research Universities
(IRU), Submission
to the Senate Community Affairs Legislation Committee, Inquiry into the Medical
Research Future Fund Bill 2015 and Medical Research Future Fund (Consequential
Amendments) Bill 2015, 13 July 2015, p. 2; The Australian Health Economics
Society (AHES), Submission
to the Senate Community Affairs Legislation Committee, Inquiry into the Medical
Research Future Fund Bill 2015 and Medical Research Future Fund (Consequential
Amendments) Bill 2015, 13 July 2015, p. 2; Notre Dame, Submission
to the Senate Community Affairs Legislation Committee, Inquiry into the Medical
Research Future Fund Bill 2015 and Medical Research Future Fund (Consequential
Amendments) Bill 2015, op. cit., pp. 1–2; MRFFAG, Submission
to the Senate Community Affairs Legislation Committee, Inquiry into the Medical
Research Future Fund Bill 2015 and Medical Research Future Fund (Consequential
Amendments) Bill 2015, op. cit., p. 3; GO8, Submission
to the Senate Community Affairs Legislation Committee, Inquiry into the Medical
Research Future Fund Bill 2015 and Medical Research Future Fund (Consequential
Amendments) Bill 2015, op. cit., pp. 2–5, all accessed 25 July 2015.
[34]. National Health and
Medical Research Council Act 1992 section 4, accessed 16 June
2015.
[35]. C
King, ‘Second
reading speech: Medical Research Future Fund Bill 2015’ , House of
Representatives, Debates, 4 June 2015, p. 5850, accessed 16 June 2015.
[36]. Ibid.,
p. 5055.
[37]. The
University of Melbourne, Submission
to the Senate Community Affairs Legislation Committee, Inquiry into the Medical
Research Future Fund Bill 2015 and Medical Research Future Fund (Consequential
Amendments) Bill 2015, op. cit., p. 4.
[38]. Ibid.
[39]. AHES,
Submission
to the Senate Community Affairs Legislation Committee, Inquiry into the Medical
Research Future Fund Bill 2015 and Medical Research Future Fund (Consequential
Amendments) Bill 2015, op. cit., p. 2.
[40]. The
Go8 is a network of Australia’s leading research-intensive universities.
[41]. GO8,
Submission
to the Senate Community Affairs Legislation Committee, Inquiry into the Medical
Research Future Fund Bill 2015 and Medical Research Future Fund (Consequential
Amendments) Bill 2015, op. cit., p. 3.
[42]. IRU,
Submission
to the Senate Community Affairs Legislation Committee, Inquiry into the Medical
Research Future Fund Bill 2015 and Medical Research Future Fund (Consequential
Amendments) Bill 2015, op. cit., p. 2; Notre Dame, Submission
to the Senate Community Affairs Legislation Committee, Inquiry into the Medical
Research Future Fund Bill 2015 and Medical Research Future Fund (Consequential
Amendments) Bill 2015, op. cit., p. 2; MRFFAG, Submission
to the Senate Community Affairs Legislation Committee, Inquiry into the Medical
Research Future Fund Bill 2015 and Medical Research Future Fund (Consequential
Amendments) Bill 2015, op. cit., p. 3.
[43]. Innovative
Research Universities (IRU), Submission
to the Senate Community Affairs Legislation Committee, Inquiry into the Medical
Research Future Fund Bill 2015 and Medical Research Future Fund (Consequential
Amendments) Bill 2015, 13 July 2015, p. 2; The Australian Health Economics
Society (AHES), Submission
to the Senate Community Affairs Legislation Committee, Inquiry into the Medical
Research Future Fund Bill 2015 and Medical Research Future Fund (Consequential
Amendments) Bill 2015, 13 July 2015, p. 2; Notre Dame, Submission
to the Senate Community Affairs Legislation Committee, Inquiry into the Medical
Research Future Fund Bill 2015 and Medical Research Future Fund (Consequential
Amendments) Bill 2015, op. cit., pp. 1–2; MRFFAG, Submission
to the Senate Community Affairs Legislation Committee, Inquiry into the Medical
Research Future Fund Bill 2015 and Medical Research Future Fund (Consequential
Amendments) Bill 2015, op. cit., p. 3, all accessed 25 July 2015.
[44]. Clause
5.
[45]. National
Health and Medical Research Council, ‘Australian
clinical trials: phases of clinical trials’, Australian Clinical Trials
website, n.d., accessed 7 August 2015.
[46]. Notre
Dame, Submission
to the Senate Community Affairs Legislation Committee, Inquiry into the Medical
Research Future Fund Bill 2015 and Medical Research Future Fund (Consequential
Amendments) Bill 2015, op. cit., p. 2.
[47]. Ibid.
[48]. MRFFAG,
Submission
to the Senate Community Affairs Legislation Committee, Inquiry into the Medical
Research Future Fund Bill 2015 and Medical Research Future Fund (Consequential
Amendments) Bill 2015, op. cit., p. 3: ‘medical innovation
means the application, commercialisation and translation of medical research
into new or better ways to improve the health and wellbeing of individuals and
the community’.
[49]. For
example, IRU, Submission
to the Senate Community Affairs Legislation Committee, Inquiry into the Medical
Research Future Fund Bill 2015 and Medical Research Future Fund (Consequential
Amendments) Bill 2015, op. cit., p. 2.
[50]. Australian Charities
and Not-for-profits Commission Act 2012, column 1, item 1 of the
table in subsection 25-5(5), accessed 8 August 2015.
[51]. Public Governance,
Performance and Accountability Act 2013, section 8 (definitions
of corporate Commonwealth entity, Commonwealth entity and listed entity),
subsection 10(1) and paragraph 11(a), accessed 8 August 2015.
[52]. Explanatory
Memorandum, Public Governance, Performance and Accountability Bill 2013, p.
18, accessed 8 August 2015.
[53]. R
Finkelstein and D Hamer, eds, LexisNexis concise Australian legal dictionary,
5th edn, LexisNexis Butterworths, Chatswood, 2011, p. 160.
[54]. Ibid.
[55]. Senate
Standing Committee on Legal and Constitutional Affairs, The
doctrine of the Shield of the Crown, Commonwealth of Australia, Canberra,
December 1992, p. 8, accessed 16 June 2015.
[56]. See
for example: National
Broadband Network Companies Act 2011, section 6, accessed 29
July 2015.
[57]. Explanatory
Memorandum, Medical
Research Future Fund Bill 2015, op. cit., p. 8.
[58]. See
also clauses 11 and 14.
[59]. Nation-building Funds
Act 2008, Chapter 4 generally. See also clauses
12 and 13 of the Bill. For information on the HHF see: Department of
Health, ‘Health and Hospitals Fund (HHF)’,
accessed 8 August 2015.
[60]. See
also clause 15A.
[61]. See
also subclause 15A(2).
[62]. See
clause 17.
[63]. See
clause 20 and paragraph 17(a). For background information on the
COAG Reform Fund and the COAG
Reform Fund Act 2008 see: R Webb, COAG
Reform Fund Bill 2008, Bills digest, 54, 2008–09, Parliamentary
Library, Canberra, 2008.
[64]. See
clause 25 and paragraph 17(b).
[65]. See
clause 29 and paragraph 17(c).
[66]. See
paragraphs 18(d)-(g) and clause 19.
[67]. Clause
14, note.
[68]. Australian
Government, ‘Medical
Research Future Fund’, Budget Overview, 2014-15, op. cit.
[69]. The
HHF is established by section 214 of the Nation-building Funds Act 2008.
[70]. Subclause
12(2).
[71]. The
Explanatory Memorandum to the Bill notes that ‘These cash amounts will be used
to meet commitments related to previously approved Health and Hospitals Fund
projects under an appropriation to be established by the Medical Research
Future Fund (Consequential Amendments) Bill 2015’: Explanatory Memorandum, Medical
Research Future Fund Bill 2015, op. cit., p. 9.
[72]. Defined
in clause 5 as the Treasurer and the Finance Minister.
[73]. Subclause
15(1); clauses 60 and 61.
[74]. Subclause
15(3); Explanatory Memorandum, Medical
Research Future Fund Bill 2015, op. cit., p. 10.
[75]. ASMR,
Submission
to the Senate Community Affairs Legislation Committee, Inquiry into the Medical
Research Future Fund Bill 2015 and Medical Research Future Fund (Consequential
Amendments) Bill 2015, op. cit., p. 3; Orygen, Submission
to the Senate Community Affairs Legislation Committee, Inquiry into the Medical
Research Future Fund Bill 2015 and Medical Research Future Fund (Consequential
Amendments) Bill 2015, op. cit., p. 3; The Australian Academy of
Technological Sciences and Engineering (ATSE), Submission
to the Senate Community Affairs Legislation Committee, Inquiry into the Medical
Research Future Fund Bill 2015 and Medical Research Future Fund (Consequential
Amendments) Bill 2015, July 2015, p. 2; Victorian Government, Submission
to the Senate Community Affairs Legislation Committee, Inquiry into the Medical
Research Future Fund Bill 2015 and Medical Research Future Fund (Consequential
Amendments) Bill 2015, op. cit., p. 2; MRFFAG, Submission
to the Senate Community Affairs Legislation Committee, Inquiry into the Medical
Research Future Fund Bill 2015 and Medical Research Future Fund (Consequential
Amendments) Bill 2015, op. cit., p. 5; GO8, Submission
to the Senate Community Affairs Legislation Committee, Inquiry into the Medical
Research Future Fund Bill 2015 and Medical Research Future Fund (Consequential
Amendments) Bill 2015, op. cit., p. 2; IRU, Submission
to the Senate Community Affairs Legislation Committee, Inquiry into the Medical
Research Future Fund Bill 2015 and Medical Research Future Fund (Consequential
Amendments) Bill 2015, op. cit., p. 3; The University of Melbourne, Submission
to the Senate Community Affairs Legislation Committee, Inquiry into the Medical
Research Future Fund Bill 2015 and Medical Research Future Fund (Consequential
Amendments) Bill 2015, op. cit., pp. 2–3, all accessed 25 July 2015.
[76]. National Health and
Medical Research Council Act 1992, paragraphs 7(1)(a)(iv), (b)
and (c), subsection 51(2).
[77]. Explanatory
Memorandum, Medical
Research Future Fund Bill 2015, op. cit., p. 14.
[78]. C
King, ‘Second
reading speech: Medical Research Future Fund Bill 2015’, House of
Representatives, Debates, 4 June 2015, p. 5850–5851, accessed 16 June
2015; See also C King, Abbott
must ensure MRFF cannot be used as a $20 billion Coalition Fund,
media release, 4 June 2015, accessed 15 June 2015; D Harrison, ‘Labor
says Medical Research Future Fund could be diverted for “pet projects"’,
The Canberra Times, (online edition), 5 June 2015, accessed 19 June
2015.
[79]. S
Ley, ‘Consideration
in detail: Medical Research Future Fund Bill 2015’, House of
Representatives, Debates, 22 June 2015, p. 7029, accessed 13 July
2015.
[80] S
Ley, ‘Consideration
in detail: Medical Research Future Fund Bill 2015’, House of
Representatives, Debates, op. cit., p. 7029.
[81]. S
Ley, ‘Consideration
in detail: Medical Research Future Fund Bill 2015’, House of
Representatives, Debates, op. cit., p. 7029; see also Supplementary
Explanatory Memorandum, Medical
Research Future Fund Bill 2015, p. 2, accessed 13 July 2015.
[82]. Supplementary
Explanatory Memorandum, op. cit., p. 3. Any such debit must be made in in
accordance with Part 2, Division 4, subdivision C, D or E of the Bill.
[83]. Ibid.
[84]. Ibid.,
pp. 3–4.
[85]. Ibid.,
pp. 2–4.
[86]. Ibid.,
p. 6.
[87]. ASMR,
Submission
to the Senate Community Affairs Legislation Committee, Inquiry into the Medical
Research Future Fund Bill 2015 and Medical Research Future Fund (Consequential
Amendments) Bill 2015, op. cit., pp. 3–4; Orygen, Submission
to the Senate Community Affairs Legislation Committee, Inquiry into the Medical
Research Future Fund Bill 2015 and Medical Research Future Fund (Consequential
Amendments) Bill 2015, op. cit., p. 3; ATSE, Submission
to the Senate Community Affairs Legislation Committee, Inquiry into the Medical
Research Future Fund Bill 2015 and Medical Research Future Fund (Consequential
Amendments) Bill 2015, July 2015, p. 2; Victorian Government, Submission
to the Senate Community Affairs Legislation Committee, Inquiry into the Medical
Research Future Fund Bill 2015 and Medical Research Future Fund (Consequential
Amendments) Bill 2015, op. cit., p. 2; MRFFAG, Submission
to the Senate Community Affairs Legislation Committee, Inquiry into the Medical
Research Future Fund Bill 2015 and Medical Research Future Fund (Consequential
Amendments) Bill 2015, op. cit., p. 5; GO8, Submission
to the Senate Community Affairs Legislation Committee, Inquiry into the Medical
Research Future Fund Bill 2015 and Medical Research Future Fund (Consequential
Amendments) Bill 2015, op. cit., p. 2; The University of Melbourne, Submission
to the Senate Community Affairs Legislation Committee, Inquiry into the Medical
Research Future Fund Bill 2015 and Medical Research Future Fund (Consequential
Amendments) Bill 2015, op. cit., pp. 2–3, all accessed 25 July 2015.
[88]. Victorian
Government, Submission
to the Senate Community Affairs Legislation Committee, Inquiry into the Medical
Research Future Fund Bill 2015 and Medical Research Future Fund (Consequential
Amendments) Bill 2015, 16 July 2015, p. 2; Universities Australia (UA), Submission
to the Senate Community Affairs Legislation Committee, Inquiry into the Medical
Research Future Fund Bill 2015 and Medical Research Future Fund (Consequential
Amendments) Bill 2015, 10 July 2015, p. 2 (both accessed 25 July
2015); UTAS, Submission
to the Senate Community Affairs Legislation Committee, Inquiry into the Medical
Research Future Fund Bill 2015 and Medical Research Future Fund (Consequential
Amendments) Bill 2015, op. cit., p. 2; UWS, Submission
to the Senate Community Affairs Legislation Committee, Inquiry into the Medical
Research Future Fund Bill 2015 and Medical Research Future Fund (Consequential
Amendments) Bill 2015, op. cit., p. 1.
[89]. UA,
Submission
to the Senate Community Affairs Legislation Committee, Inquiry into the Medical
Research Future Fund Bill 2015 and Medical Research Future Fund (Consequential
Amendments) Bill 2015, op. cit., p. 2.
[90]. Victorian
Government, Submission
to the Senate Community Affairs Legislation Committee, Inquiry into the Medical
Research Future Fund Bill 2015 and Medical Research Future Fund (Consequential
Amendments) Bill 2015, op. cit., p. 2
[91]. Paragraphs
18(a) and (c).
[92]. Paragraph
18(f).
[93]. Paragraph
18(b).
[94]. See
also: clause 14 and the discussion under the heading ‘Establishment of the Fund and Special Account’.
[95]. The
related legislation includes the Future Fund Act 2006, Nation-building
Funds Act 2008 and DisabilityCare Australia Fund Act 2013.
[96]. Subclause
20(5).
[97]. Medical
Research Future Fund Bill 2015, [Opposition
proposed amendments], items 9 to 11.
See also clause 30A at item 21 of the Opposition’s proposed amendments,
which provides that the CEO of the NHMRC has the function of giving directions
and entering into agreements in accordance with Part 2, Division 4 of the Bill
(instead of a Minister).
[98]. Clause
24.
[99]. Clause
23, note.
[100]. Subclause
25(5).
[101]. Explanatory
Memorandum, Medical
Research Future Fund Bill 2015, op. cit., p. 13.
[102]. Note
1 to subclause 26(1); clause 58.
[103]. Subclause
29(4).
[104]. Medical
Research Future Fund Bill 2015, proposed
amendments (non-government), items 18 to
20. See also clause 30A proposed by item 21, which provides
that the CEO of the NHMRC has the function of giving directions and entering
into agreements in accordance with Part 2, Division 4 of the Bill (instead of a
Minister).
[105]. Explanatory
Memorandum, Medical
Research Future Fund Bill 2015, op. cit., p. 15.
[106]. Medical
Research Future Fund Bill 2015, Government amendments [sheet
HK145].
[107]. Clause
32A.
[108]. As
noted in the Supplementary Explanatory Memorandum, Medical
Research Future Fund Bill 2015, op. cit., p. 9: ‘These Ministerial
directions are administrative rather than legislative in nature and are
therefore not a legislative instrument for the purposes of the Legislative
Instruments Act’.
[109]. The
NHMRC Strategy is prepared in accordance with paragraph 16(2)(c) of the National
Health and Medical Research Council Act 1992.
[110]. S
Ley, ‘Consideration
in detail: Medical Research Future Fund Bill 2015’, House of
Representatives, Debates, 22 June 2015, p. 7029, accessed 13 July 2015.
[111]. National
Health and Medical Research Council Act 1992, section 16.
[112]. Ibid.,
paragraphs 16(2)(a) and (c).
[113]. Ibid.,
subsection 16(1).
[114]. National Health and
Medical Research Council Act 1992, Part 7 (generally); National
Health Medical Research Council (NHMRC), ‘Funding’,
NHMRC website, accessed 10 August 2015.
[115]. National
Health and Medical Research Council Act 1992, Part 7.
[116]. The
Research Committee established by the National Health and Medical Research
Council Act 1992 advises and makes recommendations to the NHMR Council on
the application and monitoring of the MREA: subsection 35(2).
[117]. S
Ley, ‘Consideration
in detail: Medical Research Future Fund Bill 2015’ , House of
Representatives, Debates, op. cit., p. 7029. See also: Supplementary
Explanatory Memorandum, Medical
Research Future Fund Bill 2015, op. cit., p. 2: ‘The MRFF will complement
the Medical Research Endowment Account operated by the National Health and
Medical Research Council (NHMRC), and leverage the existing capabilities of the
NHMRC, including peer review, grants management, and the provision of expert
advice.’
[118]. UTAS,
Submission
to the Senate Community Affairs Legislation Committee, Inquiry into the Medical
Research Future Fund Bill 2015 and Medical Research Future Fund (Consequential
Amendments) Bill 2015, op. cit., p. 1.
[119]. Supplementary
Explanatory Memorandum, Medical
Research Future Fund Bill 2015, op. cit., p. 3.
[120]. Supplementary
Explanatory Memorandum, Medical
Research Future Fund Bill 2015, op. cit., p. 9: ‘the Strategy and
Priorities cannot be repealed, rescinded, revoked, amended, or varied in any
way under the Acts Interpretation Act. It is intended that the Strategy and
Priorities remain unchanged for periods of five and two years respectively to
support a consistent and planned approach to medical research funding.’
[121]. Supplementary
Explanatory Memorandum, Medical
Research Future Fund Bill 2015, op. cit., p. 9.
[122]. Ibid.
[123]. Subclause
15A(2).
[124]. S
Ley, ‘Consideration
in detail: Medical Research Future Fund Bill 2015’’, House of
Representatives, Debates, 22 June 2015, p. 7029, accessed 13 July 2015;
see also Supplementary Explanatory Memorandum, Medical
Research Future Fund Bill 2015, op. cit., p. 3.
[125]. Supplementary
Explanatory Memorandum, Medical
Research Future Fund Bill 2015, op. cit., p. 3.
[126]. Supplementary
Explanatory Memorandum, Medical
Research Future Fund Bill 2015, op. cit., p. 9: ‘the Strategy and
Priorities cannot be repealed, rescinded, revoked, amended, or varied in any
way under the Acts Interpretation Act. It is intended that the Strategy and
Priorities remain unchanged for periods of five and two years respectively to
support a consistent and planned approach to medical research funding.’
[127]. Supplementary
Explanatory Memorandum, Medical
Research Future Fund Bill 2015, op. cit., p. 9.
[128]. Ibid.
[129]. MRFFAG,
Submission
to the Senate Community Affairs Legislation Committee, Inquiry into the Medical
Research Future Fund Bill 2015 and Medical Research Future Fund (Consequential
Amendments) Bill 2015, op. cit., p. 4.
[130]. Professor
Alan Pettigrew, Submission
to the Senate Community Affairs Legislation Committee, Inquiry into the Medical
Research Future Fund Bill 2015 and Medical Research Future Fund (Consequential
Amendments) Bill 2015, no date, p. 2, accessed 25 July 2015.
[131]. Ibid;
Orygen, Submission
to the Senate Community Affairs Legislation Committee, Inquiry into the Medical
Research Future Fund Bill 2015 and Medical Research Future Fund (Consequential
Amendments) Bill 2015, op. cit., p. 3; ACTA, Submission
to the Senate Community Affairs Legislation Committee, Inquiry into the Medical
Research Future Fund Bill 2015 and Medical Research Future Fund (Consequential
Amendments) Bill 2015, op. cit., p. 3.
[132]. UTAS,
Submission
to the Senate Community Affairs Legislation Committee, Inquiry into the Medical
Research Future Fund Bill 2015 and Medical Research Future Fund (Consequential
Amendments) Bill 2015, op. cit., p. 2.
[133]. ACTA,
Submission
to the Senate Community Affairs Legislation Committee, Inquiry into the Medical
Research Future Fund Bill 2015 and Medical Research Future Fund (Consequential
Amendments) Bill 2015, op. cit., p. 3; ASMR, Submission
to the Senate Community Affairs Legislation Committee, Inquiry into the Medical
Research Future Fund Bill 2015 and Medical Research Future Fund (Consequential
Amendments) Bill 2015, July 2015, op. cit., p. 3.
[134]. Subclause
32G(3).
[135]. Subclause
32G(2). Whilst the text of the Bill only refers to ‘commercialisation’, the
Supplementary Explanatory Memorandum notes on page 10 that ‘Members of the
Advisory Board must... possess an appropriate balance of experience and knowledge
in.... commercialisation of research and innovation.’
[136]. Subclauses
32G(1) and (4).
[137]. Note
to subclause 32G(4) (referring to section 33AA of the Acts
Interpretation Act 1901).
[138]. Australian
Clinical Trials Alliance (ACTA), Submission
to the Senate Community Affairs Legislation Committee, Inquiry into the Medical
Research Future Fund Bill 2015 and Medical Research Future Fund (Consequential
Amendments) Bill 2015, 14 July 2015, p. 3; The Council of Academic Public
Health Institutions (CAPHIA), Submission
to the Senate Community Affairs Legislation Committee, Inquiry into the Medical
Research Future Fund Bill 2015 and Medical Research Future Fund (Consequential
Amendments) Bill 2015, 10 July 2015, p. 2; Group of Eight Australia (GO8), Submission
to the Senate Community Affairs Legislation Committee, Inquiry into the Medical
Research Future Fund Bill 2015 and Medical Research Future Fund (Consequential
Amendments) Bill 2015, 10 July 2015, p. 2; University of Western Sydney
(UWS), Submission
to the Senate Community Affairs Legislation Committee, Inquiry into the Medical
Research Future Fund Bill 2015 and Medical Research Future Fund (Consequential
Amendments) Bill 2015, no date, pp. 1 and 3, Australian Academy of Science
(AAoS), Submission
to the Senate Community Affairs Legislation Committee, Inquiry into the Medical
Research Future Fund Bill 2015 and Medical Research Future Fund (Consequential
Amendments) Bill 2015, July 2015, p. 2 (all accessed 25 July 2015).
[139]. UWS,
Submission
to the Senate Community Affairs Legislation Committee, Inquiry into the Medical
Research Future Fund Bill 2015 and Medical Research Future Fund (Consequential
Amendments) Bill 2015, op. cit., p. 3.
[140]. The
Australian Society for Medical Research (ASMR), Submission
to the Senate Community Affairs Legislation Committee, Inquiry into the Medical
Research Future Fund Bill 2015 and Medical Research Future Fund (Consequential
Amendments) Bill 2015, July 2015, p. 3, accessed 25 July 2015.
[141]. UWS,
Submission
to the Senate Community Affairs Legislation Committee, Inquiry into the Medical
Research Future Fund Bill 2015 and Medical Research Future Fund (Consequential
Amendments) Bill 2015, op. cit., p. 3; AAoS, Submission
to the Senate Community Affairs Legislation Committee, Inquiry into the Medical
Research Future Fund Bill 2015 and Medical Research Future Fund (Consequential
Amendments) Bill 2015, op. cit., p. 2; Cancer Voices Australia (CVA), Submission
to the Senate Community Affairs Legislation Committee, Inquiry into the Medical
Research Future Fund Bill 2015 and Medical Research Future Fund (Consequential
Amendments) Bill 2015, 7 July 2015, p. 2; Rare Voices Australia (RVO), Submission
to the Senate Community Affairs Legislation Committee, Inquiry into the Medical
Research Future Fund Bill 2015 and Medical Research Future Fund (Consequential
Amendments) Bill 2015, 14 July 2015, p. 2; Orygen - The National Centre for
Excellence in Youth Mental Health (Orygen), Submission
to the Senate Community Affairs Legislation Committee, Inquiry into the Medical
Research Future Fund Bill 2015 and Medical Research Future Fund (Consequential
Amendments) Bill 2015, no date, pp. 2–3; The University of Notre Dame
Australia (Notre Dame), Submission
to the Senate Community Affairs Legislation Committee, Inquiry into the Medical
Research Future Fund Bill 2015 and Medical Research Future Fund (Consequential
Amendments) Bill 2015, 10 July 2015, pp. 2–3; The Medical Research Future
Fund Action Group (MRFFAG), Submission
to the Senate Community Affairs Legislation Committee, Inquiry into the Medical
Research Future Fund Bill 2015 and Medical Research Future Fund (Consequential
Amendments) Bill 2015, no date, p. 4, all accessed 25 July 2015.
[142]. Notre
Dame, Submission
to the Senate Community Affairs Legislation Committee, Inquiry into the Medical
Research Future Fund Bill 2015 and Medical Research Future Fund (Consequential
Amendments) Bill 2015, op. cit., p. 3.
[143]. CVA,
Submission
to the Senate Community Affairs Legislation Committee, Inquiry into the Medical
Research Future Fund Bill 2015 and Medical Research Future Fund (Consequential
Amendments) Bill 2015, op. cit., p. 2.
[144]. The
use of the phrase ‘other members’ implies that the CEO of the NHMRC is
also a ‘member’ of the Advisory Board. In contrast, if the clause had read ‘the
Advisory Board is comprised of the CEO and up to seven members’, it could have
been inferred that the CEO was not a ‘member’ even though they were part of the
Advisory Board.
[145]. See
also subclauses 32H(2), (3) and (4).
[146]. See:
National Health and Medical Research Council Act 1992, section 43 and Remuneration Tribunal
Act 1973, subsections 3(1) (definitions of allowance and public office,
3(2) (definition of remuneration includes reference to annual allowances), 3(4)
(definition of ‘public office’ refers to ‘an appointment made under a law of
the Commonwealth’), 7(3) (the Remuneration Tribunal determines the remuneration
(and therefore annual allowances) to be paid to holders of public office).
[147]. Subclause
32K(1).
[148]. Subclause
32K(2), compare to: National Health and Medical Research Council Act
1992, subsections 42A(1), (3) and Public Governance,
Performance and Accountability Act 2013, sections 29 and 31.
[149]. Subclause
32K(3), compare to: Fair
Work (Registered Organisations) Bill 2014 [No. 2], proposed section 293C
(in particular proposed subsection 293C(6)).
[150]. Subclause
32K(4), compare to: National Health and Medical Research Council Act
1992, paragraph 44B(3)(b) and Public Governance, Performance and
Accountability Act 2013, section 30.
[151]. Clause
33.
[152]. See
subclauses 34(2) and (3): the Minister must give the FFB at least
90 days to prepare the MAD determination.
[153]. Paragraph
34(8)(b).
[154]. Subparagraph
34(4)(a)(i).
[155]. Subparagraph
34(4)(a)(ii).
[156]. Paragraph
34(4)(b).
[157]. Paragraph34(4)(c).
[158]. Paragraph
34(4)(d).
[159]. Paragraph
34(4)(e) and subclauses 34(5) and (6). However, those matters
must not be inconsistent with the matters to be considered under subclause
34(4), the Investment Mandate or any other provision of the Bill and must
be provided at least 90 days before the FFB is required to determine the MAD
for the next financial year: subclauses 34(6) and (7).
[160]. Explanatory
Memorandum, Medical
Research Future Fund Bill 2015, op. cit., p. 16.
[161]. Subclauses
34(9) and (10).
[162]. Subclause
37(1).
[163]. Subclauses
37(2) and (3).
[164]. See:
clause 5, which provides that the definition of a ‘financial asset’ is
that contained in the Future Fund Act 2006 (see section 6 and the
definitions of GFS Australia and GFS System in section 5 of that Act).
[165]. Australian
Bureau of Statistics (ABS), Australian
GFS Framework, Australian System of Government Finance Statistics:
Concepts, Sources and Methods, cat. no. 5514.0.55.001, ABS, Canberra, 2005,
accessed 7 August 2015.
[166]. See
for example paragraph 47(1)(a).
[167]. Explanatory
Memorandum, Medical
Research Future Fund Bill 2015, op. cit., p. 24.
[168]. Ibid.
[169]. Subclauses
50(3) and (4).
[170]. See
also: Explanatory Memorandum, Medical
Research Future Fund Bill 2015, op. cit., p. 18, which notes that subclause
36(1) ‘is intended to reinforce that amounts are invested by the Future
Fund Board for the main object of enhancing the Commonwealth’s ability to
provide grants for medical research and innovation.’
[171]. Ibid.,
p. 18.
[172]. Subclause
39(5).
[173]. Subclause
41(1).
[174]. Future
Fund Act 2006, section 18A. See also the discussion under the heading Part
2, ‘Division 4’.
[175]. Explanatory
Memorandum, Medical
Research Future Fund Bill 2015, op. cit., p. 19.
[176]. Ibid.
[177]. Subclause
39(1).
[178]. Subclause
39(2).
[179]. See
subclause 39(7), notes 1 and 2.
[180]. Clause
42 and subclause 39(7). In contrast, section 19 of the Future Fund Act
2006 requires that the Minister must give a draft copy of the Investment
Mandate to the FFB, invite their feedback and consider its response.
[181]. This
clause is expressed in nearly identical terms to section 18A of the Future
Fund Act 2006, which was inserted by the Higher Education Endowment Fund
(Consequential Amendments) Act 2007. In the second reading speech to that
Act’s Bill, ‘responsible governance’ and preventing ‘the responsible ministers
from issuing a ministerial direction that has the effect of requiring the board
to use the assets of the fund to support a particular business entity, a
particular activity or a particular business’ were cited as the reasons for making
that amendment: J Bishop, ‘Second
reading speech: Higher Education Endowment Fund (Consequential Amendments) Bill
2007’, House of Representatives, Debates, 16 August 2007, pp. 3–4,
accessed 19 June 2015.
[182]. Explanatory
Memorandum, Medical
Research Future Fund Bill 2015, op. cit., p. 23.
[183]. Subclauses
46(1), (2), and (7). However, it is worth noting that subclause
46(8) provides failing to comply with a policy does not affect the validity
of any transaction.
[184]. Explanatory
Memorandum, Medical
Research Future Fund Bill 2015, op. cit., p. 23.
[185]. Orygen,
Submission
to the Senate Community Affairs Legislation Committee, Inquiry into the Medical
Research Future Fund Bill 2015 and Medical Research Future Fund (Consequential
Amendments) Bill 2015, op. cit., p. 3; MRFFAG, Submission
to the Senate Community Affairs Legislation Committee, Inquiry into the Medical
Research Future Fund Bill 2015 and Medical Research Future Fund (Consequential
Amendments) Bill 2015, op. cit., p. 6.
[186]. Orygen,
Submission
to the Senate Community Affairs Legislation Committee, Inquiry into the Medical
Research Future Fund Bill 2015 and Medical Research Future Fund (Consequential
Amendments) Bill 2015, op. cit., p. 3.
[187]. MRFFAG,
Submission
to the Senate Community Affairs Legislation Committee, Inquiry into the Medical
Research Future Fund Bill 2015 and Medical Research Future Fund (Consequential
Amendments) Bill 2015, op. cit., p. 6.
[188]. Ibid.
[189]. Future
Fund Act 2006, sections 54 and 55.
[190]. Subclause
57A(2).
[191]. Victorian
Government, Submission
to the Senate Community Affairs Legislation Committee, Inquiry into the Medical
Research Future Fund Bill 2015 and Medical Research Future Fund (Consequential
Amendments) Bill 2015, op. cit., p. 2;
[192]. Supplementary
Explanatory Memorandum, Medical
Research Future Fund Bill 2015, op. cit., p. 10.
[193]. See
clauses 15A and 61A.
[194]. See
clauses 60, 61 and 61A.
[195]. Clauses
61A, 15A, 26 and 27. See also: clauses 24 and 25.
[196]. Productivity
Commission (PC), Identifying
and evaluating regulation reforms, Research report, Melbourne,
December 2011, accessed 4 May 2015, p. 37.
[197]. Ibid.,
p. 72.
[198]. Whilst
section 55 of the DisabilityCare
Australia Fund Act 2013 provides a similar power, this can be
contrasted to the review provisions in the following Acts, none of which
provide a similar power: Fuel
Quality Standards Act 2000, section 72; Gene Technology Act 2000,
section 194; Product
Stewardship (Oil) Act 2000, section 36; Australian Crime
Commission Act 2002, section 61A; Legislative Instruments
Act 2003, sections 59 and 60; National Transport
Commission Act 2003, section 51; SPAM Act 2003,
section 46; Water Act
2007, section 253; Dental Benefits Act 2008,
section 68; Personal
Property Securities Act 2009, section 343; Australian Information
Commissioner Act 2010, section 33; Healthcare Identifies
Act 2010, section 35; Product Stewardship Act
2011, section 109; Defence Trade Controls
Act 2012, section 74 and the Illegal Logging
Prohibition Act 2012, section 84, all accessed 10 August 2015.
[199]. Explanatory
Memorandum, Medical
Research Future Fund Bill 2015, op. cit., p. 22.
[200]. Ibid.
[201]. Ibid.,
p. 25.
[202]. Explanatory
Memorandum, Medical
Research Future Fund (Consequential Amendments) Bill 2015, p. 4.
[203]. Ibid.
[204]. Medical
Research Future Fund (Consequential Amendments) Bill 2015, item 2;
Explanatory Memorandum, Medical
Research Future Fund (Consequential Amendments) Bill 2015, p. 6.
[205]. Medical
Research Future Fund (Consequential Amendments) Bill 2015, item 1;
Explanatory Memorandum, Medical
Research Future Fund (Consequential Amendments) Bill 2015, p. 6.
[206]. Medical
Research Future Fund (Consequential Amendments) Bill 2015, item 3;
Explanatory Memorandum, Medical
Research Future Fund (Consequential Amendments) Bill 2015, p. 6.
[207]. Proposed
paragraph 81(1E)(a).
[208]. Proposed
paragraphs 81(1E)(b)-(e).
[209]. Proposed
subsection 81(2D).
[210]. Explanatory
Memorandum, Medical
Research Future Fund (Consequential Amendments) Bill 2015, p. 8.
For copyright reasons some linked items are only available to members of Parliament.
© Commonwealth of Australia

Creative Commons
With the exception of the Commonwealth Coat of Arms, and to the extent that copyright subsists in a third party, this publication, its logo and front page design are licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Australia licence.
In essence, you are free to copy and communicate this work in its current form for all non-commercial purposes, as long as you attribute the work to the author and abide by the other licence terms. The work cannot be adapted or modified in any way. Content from this publication should be attributed in the following way: Author(s), Title of publication, Series Name and No, Publisher, Date.
To the extent that copyright subsists in third party quotes it remains with the original owner and permission may be required to reuse the material.
Inquiries regarding the licence and any use of the publication are welcome to webmanager@aph.gov.au.
Disclaimer: Bills Digests are prepared to support the work of the Australian Parliament. They are produced under time and resource constraints and aim to be available in time for debate in the Chambers. The views expressed in Bills Digests do not reflect an official position of the Australian Parliamentary Library, nor do they constitute professional legal opinion. Bills Digests reflect the relevant legislation as introduced and do not canvass subsequent amendments or developments. Other sources should be consulted to determine the official status of the Bill.
Any concerns or complaints should be directed to the Parliamentary Librarian. Parliamentary Library staff are available to discuss the contents of publications with Senators and Members and their staff. To access this service, clients may contact the author or the Library‘s Central Entry Point for referral.