Bills Digest no. 93 2013–14
PDF version [745KB]
WARNING: This Digest was prepared for debate. It reflects the legislation as introduced and does not canvass subsequent amendments. This Digest does not have any official legal status. Other sources should be consulted to determine the subsequent official status of the Bill.
Paula Pyburne
Law and Bills Digest Section
18 June 2014
Contents
History of the Bill
Purpose of the Bill
Structure of the Bill
Background
Committee consideration
Statement of Compatibility with Human Rights
Policy position of non-government parties/independents
Position of major interest groups
Financial implications
Schedule 1—key issues and provisions
Schedule 2—key issues and provisions
Concluding comments
Date introduced: 19 March 2014
House: House of Representatives
Portfolio: Agriculture
Commencement: Sections 1–3 on Royal Assent; Schedules 1 and 2 on the later of Royal Assent and 1 July 2014.
Links: The links to the Bill, its Explanatory Memorandum and second reading speech can be found on the Bill’s home page, or through http://www.aph.gov.au/Parliamentary_Business/Bills_Legislation
When Bills have been passed and have received Royal Assent, they become Acts, which can be found at the ComLaw website at http://www.comlaw.gov.au/.
The Agricultural and Veterinary Chemicals Legislation Amendment Act 2013 (2013 Amendment Act)[1] received Royal Assent on 29 June 2013.[2] One of the purposes of 2013 Amendment Act was to insert into the Agricultural and Veterinary Chemicals Code Act 1994 (AgVet Code Act)[3] a requirement that first, existing approvals and registrations of active constituents and chemical products operate for a finite period and, second, when that period elapses, a new application is to be lodged for re-approval or re-registration.[4]
The primary purpose of the Agricultural and Veterinary Chemicals Legislation Amendment (Removing Re‑approval and Re-registration) Bill 2014 (the Bill) is to remove the requirement for re-approval or re‑registration of active constituents and chemical products, by making relevant amendments to the AgVet Code Act and by making consequential amendments to:
- Agricultural and Veterinary Chemical Products (Collection of Levy) Act 1994 (Collection of Levy Act)[5] and
- 2013 Amendment Act.
The Bill is comprised of two Schedules:
- Schedule 1 of the Bill amends the Collection of Levy Act, the AgVet Code Act and the 2013 Amendment Act to remove the requirements for re-approval and re-registration and
- Schedule 2 of the Bill makes miscellaneous amendments to the Agvet Code Act and the Food Standards Australia New Zealand Act 1991 (FSANZ Act).[6]
The regulatory framework for managing pesticides and veterinary medicines in Australia is collectively referred to as the National Registration Scheme for Agricultural and Veterinary Chemicals (NRS). The NRS is a partnership between the Commonwealth and the states and territories.[7] Assessment and registration of agricultural and veterinary (agvet) chemicals, as well as control of supply activities up to the point of retail sale, is undertaken by the Australian Pesticides and Veterinary Medicines Authority (APVMA).[8] Control of the use of agvet chemicals after sale is the responsibility of individual states and territories.
The role of the APVMA is to independently evaluate the safety and performance of agvet chemicals intended for supply within Australia, ensuring that the health and safety of people, animals, crops and the environment, and Australia’s ability to trade agricultural products, are protected. All registered products must be shown to work and be safe for people and the environment. Registered products also must not unduly jeopardise Australia’s trade with other nations.[9]
The APVMA was the subject of reviews by the Australian National Audit Office[10] in 2006 and by the Productivity Commission in 2008.[11] Both of those reviews made recommendations for improvement.
In response to these reviews and to complaints about the performance of the APVMA,[12] the Australian Labor Party (ALP) promised in the lead-up to the 2010 Election that it would:
... improve the regulation of agricultural and veterinary chemicals in Australia through the APVMA [and that] ... A key focus will be on the efficient assessment of lower-risk agricultural and veterinary (agvet) chemicals while ensuring that higher-risk agvet chemicals are assessed appropriately.[13]
Following the 2010 Federal Election the ALP Government released a policy discussion paper and sought public comment.[14] The reforms outlined in the discussion paper were aimed at reducing the regulatory burdens on industry and businesses, and enhancing the APVMA’s business and operational functions.[15] Further stakeholder consultation took place when the Government issued both an initial exposure draft[16] and a revised exposure draft[17] of its proposed legislation.
The 2013 Amendment Act was the product of that extensive consultation process.[18]
Dissenting view
The originating Bill for the 2013 Amendment Act was referred to the House of Representatives Standing Committee for Agriculture, Resources, Fisheries and Forestry (the Agriculture, Resources, Fisheries and Forestry Committee) for inquiry and report.[19] Whilst the majority report of the Agriculture, Resources, Fisheries and Forestry Committee recommended that the Bill be passed,[20] the Coalition members provided a dissenting report which stated that the:
... Bill as is drafted provides a substantial increase in regulatory burden and costs that will have a negative impact on industry without significantly improving the efficiency of regulation and the re-registration process will slow down rather than increase the review of suspect chemistries. To achieve genuine efficiencies within the system that allow for a more timely review of suspect chemistries it is vital that the proposed re-registration process be removed from the bill.[21]
Similarly, the provisions of the originating Bill for the 2013 Amendment Act were referred to the Senate Rural and Regional Affairs and Transport Legislation Committee (Rural and Regional Affairs and Transport Committee) for inquiry and report.[22] Whilst the majority of the Rural and Regional Affairs and Transport Committee recommended that the Bill be passed, the Coalition Senators[23] provided a dissenting report recommending that the originating Bill should not be passed.[24]
In the lead up to the 2013 Federal Election, the Coalition signalled its intention (if elected) to reform the agriculture and veterinary chemicals legislation to improve efficiencies by, amongst other things, removing reregistration.[25] This is consistent with the Coalition’s views as expressed in relation to the corresponding terms of the 2013 Amendment Act.
This Bill delivers on that election promise.
Rural and Regional Affairs and Transport Committee
The Bill has been referred to the Rural and Regional Affairs and Transport Committee (the second Rural and Regional Affairs and Transport Committee) for inquiry and report by 16 June 2014.[26] The views of submitters are canvassed under the Key issues and provisions heading of this Bills Digest.
Senate Standing Committee for the Scrutiny of Bills
The Senate Standing Committee for the Scrutiny of Bills has no comment on the Bill.[27]
Parliamentary Joint Committee on Human Rights
At its meetings of 25 March 2014 and 14 May 2014, the Parliamentary Joint Committee on Human Rights deferred consideration of the Bill.[28]
As required under Part 3 of the Human Rights (Parliamentary Scrutiny) Act 2011 (Cth), the Government has assessed the Bill’s compatibility with the human rights and freedoms recognised or declared in the international instruments listed in section 3 of that Act.[29] The Government considers that the Bill is compatible.
Australian Labor Party
As stated above, the 2013 Amendment Act arose from an ALP promise to improve the regulation of agricultural and veterinary chemicals in Australia in the lead-up to the 2010 Election.
The comments of the majority of members of the Rural and Regional Affairs and Transport Committee in relation to the Bill which inserted the requirements for re-approval and re-registration indicate that they considered that mandatory review of agvet chemicals should occur on a regular basis. On the other hand they recognised that an unintended consequence of mandatory review might be the loss to the market of low-value but widely used chemicals. That being the case, a review of the system was proposed after it had been in operation for five years.[30]
Australian Greens
The Australian Greens (the Greens) members of the Rural and Regional Affairs and Transport Committee in relation to the Bill which inserted the requirements for re-approval and re-registration provided a minority report stating:
... this reform is essential, but it is important that the re-registration process and subsequent reviews of chemical use achieve the ultimate goal of managing risk to human life and the environment, and are based on scientific analysis, take account of decisions made in other countries and the actions of the APVMA are not hampered in its risk assessments by a lack of data or a lack of definitional clarity.[31]
Agricultural producers and other chemical users are strongly supportive of the decision to remove the requirements for re-approval and re-registration which is considered to be an ‘unnecessary process’[32] which would ‘increase the regulatory burden on applicants, registrants and approval holders’.[33]
According to the Explanatory Memorandum to the Bill, the amendments will have no financial impact for the Budget.[34]
Re-approval and re-registration
The 2013 Amendment Act amended the AgVet Code to, amongst other things, provide for the re-approval and re-registration of active constituents and chemical products. The amendments in Schedule 1 of the Bill to remove these requirements include, but are not limited to:
- item 30 repeals Division 3A of Part 2 of the Agvet Code (clauses 29C-29K) which provides for re-approval and re-registration of active constituents and chemical products
- items 6, 8 and 9 make consequential changes to definitions in clause 3 of the Agvet Code
- items 13—17 amend the notice provisions in clause 8F of the Agvet Code to remove references to re‑approvals and re-registrations
- item 19 amends paragraph 8S(1)(b) of the Agvet Code to remove the requirement for the APVMA to give notice of a decision to re-approve or re-register an active constituent, chemical product or label
- items 22–25 amend clause 19 of the Agvet Code to remove references to the date an approval ends
- item 39 repeals and replaces subclause 47(1) of the Agvet Code so that that approval of an active constituent continues in force unless it is cancelled and
- items 44–51 amend clauses 47B and 47C of the Agvet Code to remove the requirement that the APVMA give advance notice of the end of an approval or registration.
Arguments for re-approval and re-registration
The rationale for the requirement for re-approval and re-registration was that it would:
... increase the scrutiny of chemical constituents and products through a scheme that minimises impacts on industry. The scheme provides a greater level of assurance that existing chemicals and products do not pose an undue risk to human health or the environment, and further promotes public confidence in agvet chemical regulation.[35]
WWF Australia noted that there are pesticides on the market today that have never been assessed according to today’s scientific and regulatory standards. That organisation expressed its concern that ‘by removing the re‑approval and re-registration scheme, those pesticides and products will continue to be used on the market without a contemporary risk assessment, potentially placing the community and environment at risk’.[36]
Arguments against re-approval and re-registration
Many stakeholders did not agree with the proposed re-approval and re-registration requirement at the time that it was inserted into the Agvet Code. For example, AgForce Queensland considered that it would ‘result in delays for new crop and animal protection products’.[37] CropLife described the measures as being ‘expensive, unfair and will not deliver any better protection of workers, consumers or the environment’.[38]
Submitters to the second Rural and Regional Affairs and Transport Committee which is inquiring into this Bill broadly support the repeal of the requirement for re-approval and re-registration.[39] The National Farmers’ Federation, for example, which was a ‘vocal opponent’ of the requirement for re-approval and re‑registration, welcomed the Government’s commitment to remove the process.[40]
Effectiveness of chemical review process
Some supporters of the Bill argue that there is no need for re-approval and re-registration system because the Agvet Code already provides for chemical reviews.[41] Existing clause 31 of the Agvet Code provides that the APVMA may, at any time, reconsider an approval of an active constituent for a proposed or existing chemical product, the registration of a chemical product or the approval of a label for containers of a chemical product.[42] In addition, the APVMA may invite the public to propose active constituents of chemical products, or their labels, the approval or registration of which the APVMA might reconsider.[43]
The question arises then, as to whether the chemical review process which may take place under section 31 of the Agvet Code is effective. In 2008, the Productivity Commission examined the existing arrangements for the regulation of chemicals and plastics in Australia. As part of its examination, the Productivity Commission described the conduct of chemical reviews as follows:
Where potential safety or performance risks have been identified, APVMA, as part of its Chemical Review Program (CRP), undertakes public reviews of already registered chemicals (including those assessed under the previous jurisdictional regimes) to assess whether they still work as intended and are safe for humans and the environment. Potential reviews are prioritised and scoped, based on the level of concern about possible adverse effects determined against agreed selection criteria. There have been around 100 reviews completed or underway under this program, with over 40 existing chemicals currently nominated for future review.
As existing agvet chemical products have already received some form of assessment under previous state and territory registration schemes, the problem is not as significant as for industrial chemicals. In addition, about 75 per cent of agvet chemical products were registered pre-1995, compared to around 95 per cent of grandfathered industrial chemicals.
An Australian National Audit Office audit of APVMA’s regulation of pesticides and veterinary medicines (ANAO 2006) considered that it had reasonable arrangements for identifying and prioritising existing chemicals requiring review. However, even for the relatively small subset of existing chemicals identified and prioritised for review, ANAO noted the slow rate of progress in commencing and completing reviews. It observed that this meant that the risks of using many existing chemicals remained. Hence, ANAO recommended that APVMA assess whether the time taken to complete reviews adequately incorporated the risk of delaying reviews of other products.[44]
That slow process continues.
Access to information
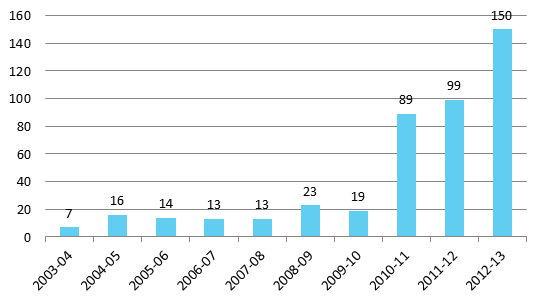
According to the Explanatory Memorandum, ‘the APVMA is often asked by companies to provide information relating to their own registered chemical products (including about the formulation and details of manufacturing)’.[45] The Table below shows the requests received by the APVMA under the Freedom of Information Act 1982,[46] in the period from 2003–04 to 2012–13.[47]
Currently information held by the APVMA is accessible under two categories:
- under the Freedom of Information Act subject to payment of the fees prescribed in the Freedom of Information (Charges) Regulations 1982[48] and subject to the condition that confidential information is not to be disclosed[49] and
- under the Agvet Code, which allows a person to apply for a copy of, or extract from, the Record of Approved Active Constituents for Chemical Products[50] or the Register of Agricultural and Veterinary Chemical Products[51] subject to payment of a prescribed fee and to the condition that confidential information is not to be disclosed.
The reason that the material on the Record or the Register is not obtained under the Freedom of Information Act is that paragraph 12(1)(b) of that Act exempts from the FOI access regime information which is open to public access, as part of a public register or otherwise, in accordance with another enactment, where that access is subject to a fee or other charge.
Item 24 of Schedule 2 of the Bill inserts proposed Division 7—Access to certain documents and information into Part 1 of the Agvet Code. Within Division 7, proposed clause 8W provides that a person may apply to the APVMA for a copy of, or extract from, a document in the possession or custody of the APVMA in relation to an approved active constituent or registered chemical product. The amendment operates so that this information is exempt from the FOI access regime. Instead, access will be provided under the Agvet Code.
Key issue—cost of access
The Department of Agriculture states that ‘the fee paid for the FOI Act request is not sufficient to cover the costs of providing this information’.[52] The amendment allows the APVMA to charge a prescribed fee which will, presumably, be higher than the fees prescribed in the Freedom of Information (Charges) Regulations. However, no details of the manner in which those fees will be calculated have been provided.
Variations
The 2013 Amendment Act amended Division 2A of Part 2 of the Agvet Code to provide for prescribed variations of the relevant particulars of an approval or registration—if the relevant particular is set out in a legislative instrument made by the APVMA. However, the Department of Agriculture doubts that it is likely to be effective in its current form.[53] Consequently, items 29 and 30 of Schedule 2 of the Bill amend the Agvet Code so that there will be two types of variations, that is, notified variations and prescribed variations.
Notified variations
Proposed Division 2AA—Notified variations of relevant particulars is inserted by item 29 of Schedule 2 of the Bill.[54] Proposed Division 2AA of Part 2 of the Agvet Code operates as follows:
- a notifiable variation is a variation of a kind that is either determined by the APVMA under subclause 26AB(5) or is prescribed by the regulations. Specifically, a notifiable variation is not a prescribed variation
- the APVMA may, by legislative instrument, determine that a kind of variation is a notifiable variation[55] if it is satisfied that with the particular so varied:
– for an active constituent—the constituent would meet the safety criteria[56]
– for a chemical product—the product would: meet the safety criteria, the trade criteria[57] and the efficacy criteria[58] or comply with the established standard for the product[59]
– for a label for a chemical product—the label would meet the labelling criteria[60] or comply with the established standard for the product.
- the holder of a registration or approval may lodge a notice with the APVMA of one or more notifiable variations of the relevant particulars of the approval or registration, provided that the notice meets the notice requirements in proposed clause 26AD[61]—in particular it must contain, or be accompanied by any information that has been prescribed by regulations or specified by the APVMA by legislative instrument
- when such a notice is lodged, the APVMA must vary the relevant particulars of the approval or registration and within 14 days update the Record,[62] Register[63] or APVMA file. The date of effect of the notice is the day on which it is lodged.[64]
Prescribed variations
Item 31 of Schedule 2 of the Bill repeals and replaces clauses 26A–26C of the Agvet Code, which are contained in Division 2A of Part 2 of the Agvet Code.[65] Amended Division 2A of Part 2 of the Agvet Code will operate as follows:
- a prescribed variation is a variation of a kind that is either determined by the APVMA under subclause 26B(6) or is prescribed by the regulations[66]
- the APVMA may, by legislative instrument, determine that a kind of variation is a prescribed variation.[67] The conditions under which such a determination may be made are set out in identical terms as those for a notifiable variation[68]
- the holder of a registration or approval may apply to the APVMA for one or more prescribed variations of the relevant particulars of the approval or registration, provided that the notice meets the application requirements in clause 8A of the Agvet Code[69]
- the APVMA must, within a period prescribed by the regulations vary the registration or approval, or refuse the application[70]—a failure to make a decision within that time is a deemed decision to vary the particulars of the registration or approval[71] and
- variation of relevant particulars takes place when the APVMA updates the Record, Register or APVMA file.[72]
Key issues—lack of detail
Submitters to the second Rural and Regional Affairs and Transport Committee are generally supportive of the Government’s commitment to reducing red tape and regulatory burdens.[73] However, according to the WWF, without seeing the list of prescribed variations which the APVMA may consider as simple variations, it is not possible for it to support the amendment.[74]
CropLife Australia commented on the absence of supporting material which would provide ‘clear guidance to allow applicants to understand what sort of variations to relevant particulars might be able to be made through this process’. It is seeking a process which is ‘as administratively simple as possible in order to encourage its use by approval holders’.[75]
Whilst the relevant Explanatory Memorandum is short on detail, the submission by the Department of Agriculture to the second Rural and Regional Affairs and Transport Committee provides the following examples:
- changes to a product’s name, perhaps because the supplier company changes hands, or to respond to market demand
- introducing smaller pack sizes where larger versions already exist
- specialising products by focussing on particular use patterns for a product with a specialised name
- changing sites of manufacture to respond to changes in the company’s supply chain and
- minor variations to chemical composition resulting from improved ingredient quality, to respond to changes in the company’s supply chain or to respond to market demand—for example, to change the scent of a personal insect repellent or change the colour of a flea collar.[76]
Product quality
Currently Part 5 of the Agvet Code sets out the procedure by which samples or substances are to be analysed and states how evidence of the results of the analysis may be given in proceedings under the Code.[77] Inspectors are appointed under subsection 69F(1) of the Agvet Administration Act or authorised under subsection 69F(2) for the purposes of the Agvet Administration Act.
Clause 97 of the Agvet Code provides that an inspector may give a portion of a sample taken under the monitoring powers[78] or the investigation powers[79] to an approved analyst for analysis.
In addition, existing clause 99 empowers the APVMA to give a notice to a person who has possession or custody of a substance (or mixture of substances) to be supplied as a chemical product under a particular name, requiring the person to have the substance or mixture analysed. The impetus for such a notice is advice that an inspector reasonably suspects that the constituents, or concentration of the constituents, of the substance or mixture, or the composition or purity of a constituent of a substance or mixture is inconsistent with the information on the Register.
According to the Explanatory Memorandum, ‘removing the requirement for re-registration removes an opportunity for the APVMA to confirm that chemical products supplied to the market are the same as the product evaluated and registered by the APVMA’.[80]
Consequently, item 43 of Schedule 2 of the Bill repeals and replaces subclauses 99(1)–(5) of the Agvet Code so that the trigger for the exercise of the powers in those subclauses is a reasonable belief by the APVMA that it is necessary for any one of the following reasons:
- to protect the health and safety of human beings
- to protect animals, plants or things, or the environment and
- to prevent significant prejudice to trade or commerce between Australia and places outside Australia.
In that case, the APVMA may give a notice, in accordance with proposed subclause 99(2) of the Agvet Code, to a person who has, has had or will have, possession or custody of a substance (or mixture of substances) to be supplied as a chemical product under a particular name requiring the person to:
- give the APVMA, within a reasonable period that is specified in the notice, information or documents as set out in proposed subclause 99(3) and/or
- within such reasonable period as specified in the notice, have the substance or mixture analysed and provide the APVMA with the results of the analysis and the analyst’s certificate: proposed subclause 99(4).[81]
Proposed subclause 99(5) of the Agvet Code provides that a person who is given a notice under subsection 99(2) of the Agvet Code must not fail to comply with the notice.
By introducing the requirements for re-approval or re-registration the former Government intended that there would be a scheme to provide a greater level of assurance that existing chemicals and products do not pose an undue risk to human health or the environment, and to promote public confidence in agvet chemical regulation.[82]
Whilst the Bill appears to have many supporters, it will be difficult for those who do not support the Bill to reconcile the decision of the current Government to repeal the requirement for re-approval or re-registration before those provisions have commenced and there has been an opportunity to assess their effect.
Members, Senators and Parliamentary staff can obtain further information from the Parliamentary Library on (02) 6277 2500.
[1]. Agricultural and Veterinary Chemicals Legislation Amendment Act 2013, accessed 25 March 2014.
[2]. Parliament of Australia, ‘Agricultural and Veterinary Chemicals Legislation Amendment Bill 2013 homepage’, Australian Parliament website, accessed 25 March 2014.
[3]. Agricultural and Veterinary Chemicals Code Act 1994, accessed 7 April 2014.
[4]. For details of the amendments see P Pyburne, Agricultural and Veterinary Chemicals Legislation Amendment Bill 2012, Bills digest, 89, 2012–13, Parliamentary Library, Canberra, 2013, accessed 7 April 2014.
[5]. Agricultural and Veterinary Chemical Products (Collection of Levy) Act 1994, accessed 25 March 2014.
[6]. Food Standards Australia New Zealand Act 1991, accessed 7 April 2014.
[7]. Australian Pesticides and Veterinary Medicines Authority (APVMA), ‘National Registration Scheme for Agricultural and Veterinary Chemicals’, APVMA website, accessed 7 April 2014.
[8]. The APVMA is established under the Agricultural and Veterinary Chemicals (Administration) Act 1992, accessed 7 April 2014.
[9]. Australian Pesticides and Veterinary Medicines Authority, Cost recovery discussion paper: covering the period 1 July 2012–30 June 2015, accessed 7 April 2014.
[10]. Australian National Audit Office, Regulation of pesticides and veterinary medicines, Audit report no. 14, 2006–07, 2006, accessed 7 April 2014.
[11]. Productivity Commission (PC), Chemicals and plastics regulation, Research report, PC, July 2008, accessed 7 April 2014.
[12]. For example, D Jopson and R Pollard, ‘Name your poison—it’s legal’, The Sydney Morning Herald, 22 October 2007, p. 1, accessed 7 April 2014; D Jopson, ‘Banned chemical on shelves despite fears it can harm health’, The Sydney Morning Herald, 22 October 2007, p. 15, accessed 7 April 2014; M Denholm, ‘Regulator stalls on review of weed killer’, The Australian, 27 June 2008, p. 8, accessed 7 April 2014.
[13]. Minister for Agriculture, Fisheries and Forestry, Gillard Labor Government will support Australia’s agricultural industries into the future, campaign media statement, 17 August 2010, p. 6, accessed 7 April 2014.
[14]. J Ludwig (Minister for Agriculture, Fisheries and Forestry), Better regulation of agricultural and veterinary chemicals, Policy discussion paper, Commonwealth of Australia, November 2010, accessed 7 April 2014.
[15]. Ibid., p. 5.
[16]. The initial draft was released for community consultation between November 2011 and February 2012.
[17]. The revised draft was released for community consultation between September 2012 and October 2012.
[18]. T Burke, Better regulation of chemicals, media release, Australian Labor Party, 14 August 2010, accessed 7 April 2014.
[19]. Details of the inquiry including the terms of reference, submissions from stakeholders and the Standing Committee for Agriculture, Resources, Fisheries and Forestry report are on the inquiry homepage, accessed 7 April 2014.
[20]. House of Representatives Standing Committee on Agriculture, Resources, Fisheries and Forestry, Advisory report on the Agricultural and Veterinary Chemicals Legislation Amendment Bill 2012, Commonwealth of Australia, Canberra, February 2013, accessed 7 April 2014.
[21]. Ibid., p. 52.
[22]. Details of the inquiry including the terms of reference, submissions from stakeholders and the Rural and Regional Affairs and Transport Committee report are on the inquiry homepage, accessed 7 April 2014.
[23]. Rural and Regional Affairs and Transport Committee, Agricultural and Veterinary Chemicals Legislation Amendment Bill 2012 [Provisions], Senate, Canberra, February 2013, pp. 27–30, accessed 16 June 2014.
[24]. Ibid., p. 30.
[25]. Liberal Party of Australia and the Nationals, The Coalition’s policy for a competitive agriculture sector, Coalition policy document, Election 2013, p. 10, accessed 7 April 2014.
[26]. Details of the terms of reference, the submissions to the Committee and the final report (when published) are on the inquiry homepage, accessed 7 April 2014.
[27]. Senate Standing Committee for the Scrutiny of Bills, Alert Digest No. 5 of 2014, Senate, Canberra, 14 May 2014, p. 1, accessed 29 May 2014.
[28]. Parliamentary Joint Committee on Human Rights, Fifth report of the 44th Parliament, The Senate, Canberra, 25 March 2014 and Parliamentary Joint Committee on Human Rights, Sixth report of the 44th Parliament, The Senate, Canberra, 14 May 2014, accessed 30 May 2014.
[29]. The Statement of Compatibility with Human Rights can be found at pages 6–8 of the Explanatory Memorandum to the Bill.
[30]. Rural and Regional Affairs and Transport Committee, Inquiry into the Agricultural and Veterinary Chemicals Legislation Amendment Bill 2012 [Provisions], Senate, Canberra, 2013, p. 15, accessed 2 June 2014.
[31]. Australian Greens Senators, Minority report, Rural and Regional Affairs and Transport Committee, Inquiry into the Agricultural and Veterinary Chemicals Legislation Amendment Bill 2012 [Provisions], Senate, Canberra, February 2013, p. 35, accessed 2 June 2014.
[32]. National Farmers’ Federation, Submission to Senate Rural and Regional Affairs and Transport Committee, Inquiry into the Agricultural and Veterinary Chemicals Legislation Amendment (Removing Re-approval and Re-registration) Bill 2014, 11 April 2014, p. 1, accessed 30 May 2014.
[33]. Pastoralists and Graziers Association of Western Australia, Submission to Senate Rural and Regional Affairs and Transport Committee, Inquiry into the Agricultural and Veterinary Chemicals Legislation Amendment (Removing Re-approval and Re-registration) Bill 2014, 11 April 2014, p. 1, accessed 30 May 2014.
[34]. Explanatory Memorandum, Agricultural and Veterinary Chemicals Legislation Amendment (Removing Re-approval and Re-registration) Bill 2014, p. 5, accessed 16 June 2014.
[35]. Explanatory Memorandum, Agricultural and Veterinary Chemicals Legislation Amendment Bill 2012, p. 3, accessed 17 June 2014.
[36]. WWF, Submission to the Rural and Regional Affairs and Transport Legislation Committee, Inquiry into the Agricultural and Veterinary Chemicals Legislation Amendment (Removing Re-approval and Re-registration) Bill 2014, 16 April 2014, p. 6, accessed 2 June 2014.
[37]. AgForce Queensland, Submission to the Rural and Regional Affairs and Transport Legislation Committee, Inquiry into the Agricultural and Veterinary Chemicals Legislation Amendment Bill 2012, 19 December 2012, p. 3, accessed 10 April 2014.
[38]. CropLife Australia, Submission to the Rural and Regional Affairs and Transport Legislation Committee, Inquiry into the Agricultural and Veterinary Chemicals Legislation Amendment Bill 2012, 19 December 2012, p. 5, accessed 11 June 2014.
[39]. Grain Producers Australia, Submission to the Rural and Regional Affairs and Transport Legislation Committee, Inquiry into the Agricultural and Veterinary Chemicals Legislation Amendment (Removing Re-approval and Re-registration) Bill 2014, 17 April 2014, p. 3, accessed 11 June 2014.
[40]. National Farmers’ Federation, Submission to the Rural and Regional Affairs and Transport Legislation Committee, op. cit., p. 1.
[41]. NSW Farmers, Submission to the Rural and Regional Affairs and Transport Legislation Committee, Inquiry into the Agricultural and Veterinary Chemicals Legislation Amendment (Removing Re-approval and Re-registration) Bill 2014, March 2014, p. 4, accessed 2 June 2014; CropLife Australia, Submission to the Rural and Regional Affairs and Transport Legislation Committee, Inquiry into the Agricultural and Veterinary Chemicals Legislation Amendment (Removing Re-approval and Re-registration) Bill 2014, 17 April 2014, p. 3, accessed 11 June 2014.
[42]. Australian Pesticides and Veterinary Medicines Authority (APVMA), ‘Chemical review programs’, APVMA website, accessed 10 April 2014.
[43]. Clause 30 of the Agvet Code.
[44]. Productivity Commission, Chemicals and plastics regulation, Research report, op. cit., p. 209.
[45]. Explanatory Memorandum, p. 19.
[46]. Freedom of Information Act 1982, accessed 10 June 2014.
[47]. Australian Pesticides and Veterinary Medicines Authority, Annual report 2012–13, APVMA, 2013, p. 166, accessed 11 June 2014.
[48]. Freedom of Information (Charges) Regulations 1982, accessed 10 June 2014.
[49]. Clause 162 of the Agvet Code.
[50]. Subclauses 17(4) and (5) of the Agvet Code.
[51]. Subclauses 18(4) and (5) of the Agvet Code.
[52]. Department of Agriculture, Submission to the Rural and Regional Affairs and Transport Legislation Committee, Inquiry into the Agricultural and Veterinary Chemicals Legislation Amendment (Removing Re-approval and Re-registration) Bill 2014, undated, p. 10, accessed 11 June 2014.
[53]. Explanatory Memorandum, p. 18.
[54]. Note that items 7–9, 12, 17, 25, 49–50 and 54 of Schedule 2 of the Bill amend the Agvet Code in relation to the proposed Division 2AA of Part 2.
[55]. Proposed subclause 26AB(5) of the Agvet Code.
[56]. The term meets the safety criteria is defined in clause 5A of the Agvet Code which was inserted by the 2013 Amendment Act. That provision is due to commence on 1 July 2014.
[57]. The term meets the trade criteria is defined in clause 5C of the Agvet Code which was inserted by the 2013 Amendment Act. That provision is due to commence on 1 July 2014.
[58]. The term meets the efficacy criteria is defined in clause 5B of the Agvet Code which was inserted by 2013 Amendment Act. That provision is due to commence on 1 July 2014.
[59]. The power of the APVMA to make standards is set out in clause 6E of the Agvet Code which was inserted by 2013 Amendment Act. That provision is due to commence on 1 July 2014.
[60]. The term meets the labelling criteria is defined in clause 5D of the Agvet Code which was inserted by 2013 Amendment Act. That provision is due to commence on 1 July 2014.
[61]. Proposed subclauses 26AB(1) and (2) of the Agvet Code.
[62]. The Record is the Record of Approved Active Constituents for Chemical Products kept under clause 17 of the Agvet Code.
[63]. The Register is the Register of Agricultural and Veterinary Chemical Products kept under clause 18 of the Agvet Code.
[64]. Proposed subclause 26AC(2) of the Agvet Code.
[65]. Note that items 10, 17, 25 and 26 of Schedule 2 of the Bill amend the Agvet Code in relation to the proposed Division 2A of Part 2.
[66]. Proposed subclause 26B(4) of the Agvet Code.
[67]. Proposed subclause 26B(4) of the Agvet Code.
[68]. Proposed paragraphs 26B(5)(a)–(c) of the Agvet Code.
[69]. Proposed subclauses 26B(1) and (2) of the Agvet Code.
[70]. Proposed subclause 26C(1) of the Agvet Code.
[71]. Proposed subclause 26C(2) of the Agvet Code.
[72]. Proposed subclause 26D(1) of the Agvet Code.
[73]. Accord, Submission to the Rural and Regional Affairs and Transport Legislation Committee, Inquiry into the Agricultural and Veterinary Chemicals Legislation Amendment (Removing Re-approval and Re-registration) Bill 2014, 11 April 2014, p. 1, accessed 12 June 2014; AgForce, Submission to the Rural and Regional Affairs and Transport Legislation Committee, Inquiry into the Agricultural and Veterinary Chemicals Legislation Amendment (Removing Re-approval and Re-registration) Bill 2014, 16 April 2014, p. 5, accessed 12 June 2014.
[74]. WWF, Submission to the Rural and Regional Affairs and Transport Legislation Committee, op. cit., p. 11.
[75]. CropLife Australia, Submission to the Rural and Regional Affairs and Transport Legislation Committee, op. cit., p. 6.
[76]. Department of Agriculture, Submission to the Rural and Regional Affairs and Transport Legislation Committee, op. cit., p. 9.
[77]. Clause 96 of the Agvet Code.
[78]. Clause 131A of the Agvet Code.
[79]. Clause 132A of the Agvet Code.
[80]. Explanatory Memorandum, p. 18.
[81]. Item 55 of Schedule 2 of the Bill amends paragraph 167(1)(j) of the Agvet Code so that a person may make an application to the Administrative Appeals Tribunal for a review of the decision to issue a notice under clause 99.
[82]. Explanatory Memorandum, Agricultural and Veterinary Chemicals Legislation Amendment Bill 2012, p. 3.
For copyright reasons some linked items are only available to members of Parliament.
© Commonwealth of Australia

Creative Commons
With the exception of the Commonwealth Coat of Arms, and to the extent that copyright subsists in a third party, this publication, its logo and front page design are licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Australia licence.
In essence, you are free to copy and communicate this work in its current form for all non-commercial purposes, as long as you attribute the work to the author and abide by the other licence terms. The work cannot be adapted or modified in any way. Content from this publication should be attributed in the following way: Author(s), Title of publication, Series Name and No, Publisher, Date.
To the extent that copyright subsists in third party quotes it remains with the original owner and permission may be required to reuse the material.
Inquiries regarding the licence and any use of the publication are welcome to webmanager@aph.gov.au.
Disclaimer: Bills Digests are prepared to support the work of the Australian Parliament. They are produced under time and resource constraints and aim to be available in time for debate in the Chambers. The views expressed in Bills Digests do not reflect an official position of the Australian Parliamentary Library, nor do they constitute professional legal opinion. Bills Digests reflect the relevant legislation as introduced and do not canvass subsequent amendments or developments. Other sources should be consulted to determine the official status of the Bill.
Any concerns or complaints should be directed to the Parliamentary Librarian. Parliamentary Library staff are available to discuss the contents of publications with Senators and Members and their staff. To access this service, clients may contact the author or the Library‘s Central Entry Point for referral.